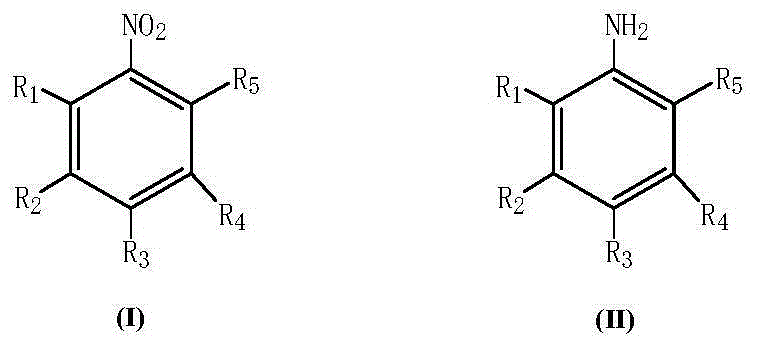
Palladium/alkali metal compound supported catalyst and preparation method and application thereof
A technology of alkali metal compounds and supported catalysts, which is applied in the direction of metal/metal oxide/metal hydroxide catalysts, amino compound preparation, organic compound preparation, etc., and can solve the difficulty of separation in the process of increasing the recovery and reuse of precious metal active components , Aggravate the environmental pollution of recycled waste liquid rich in heavy metals, weaken the catalytic hydrogenation activity of catalysts, etc., and achieve the effects of stable properties of alkali metal compounds, more times of application, and high metal utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
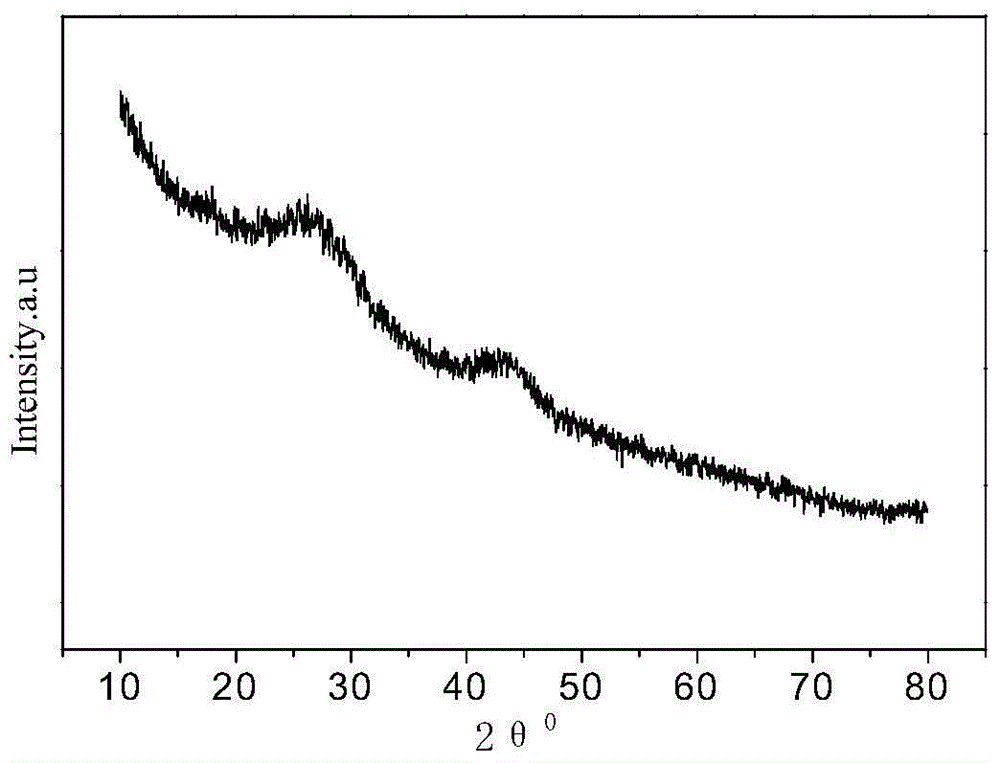
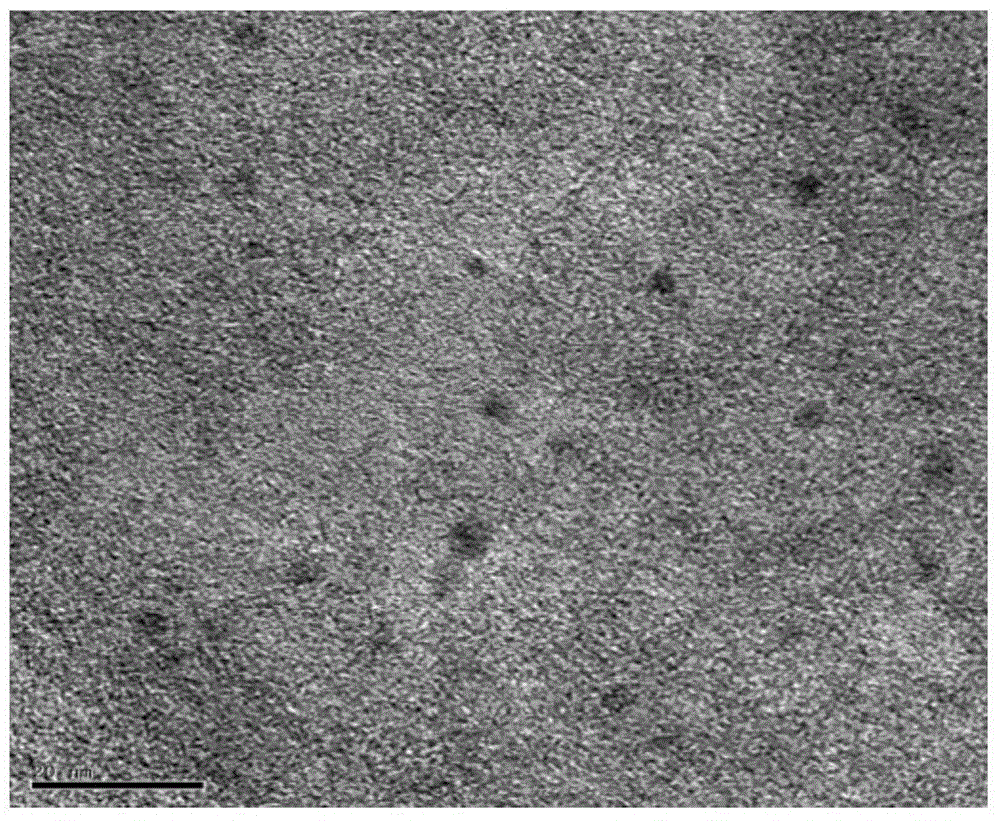
Image
Examples
Embodiment 1~ Embodiment 18
[0046] The content, proportion and preparation conditions of the catalyst active components are provided (as shown in Table 1).
[0047] Catalyst concrete preparation process is as follows (taking embodiment 1 as example):
[0048] (a) Set the specific surface area to 1400m 2 / g, 0.85mL / g pore volume, 3.2% ash content coconut shell activated carbon was vacuum-dried and dehydrated at 120°C for 3h; L) in 50mL deionized water, adjust the pH value to 0.8 with hydrochloric acid;
[0049] (b) then immerse 10 g of activated carbon that has been vacuum-dried and dehydrated in palladium liquid, fully stir and impregnate at 80° C. for 5 hours, and adjust the pH value to 9.5 with ammonia water;
[0050] (c) After half an hour, 1.5 mL of formaldehyde solution (GB-T685-1993) was added dropwise, and reduced at 80° C. for 2 h. After cooling down to room temperature, the reaction system was filtered, the filter cake was washed with deionized water until neutral, and dried and dehydrated at...
Embodiment 19
[0066]Add 200g of 6-chloro-2-nitrotoluene and 1.0g of the palladium / alkali metal compound supported catalyst prepared in Example 1 into a 500mL autoclave, close the autoclave, replace the air in the reactor with nitrogen, and then replace it with hydrogen Nitrogen, start stirring, stirring rotation speed 1000r / min, maintain reaction temperature 50°C, hydrogen pressure 0.5MPa to carry out the reaction. When the content of 6-chloro-2-nitrotoluene was detected to be 0 by chromatography, the reaction was stopped and the catalyst was filtered. The filtrate is the product after phase separation, water separation and dehydration by vacuum distillation. Quantitative analysis (molar percentage) by chromatography shows that the conversion rate of hydrogenation reaction is 100%, and the selectivity is 99.96%.
[0067] The operating condition of embodiment 20~embodiment 39 is the same as embodiment 19, is respectively the hydrogenation catalyst that the embodiment 2~embodiment 18 and comp...
Embodiment 58
[0076] Example 58 is the experimental situation of using the catalyst prepared in Example 2 in the catalytic hydrogenation reaction of 3,4-dichloronitrobenzene under the operating conditions of Example 19. The results are shown in Table 4.
[0077] Catalyst prepared in the embodiment 2 of table 4 synthesizes 3, the mechanically applied experimental result of 4-dichloroaniline reaction
[0078]
[0079]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com