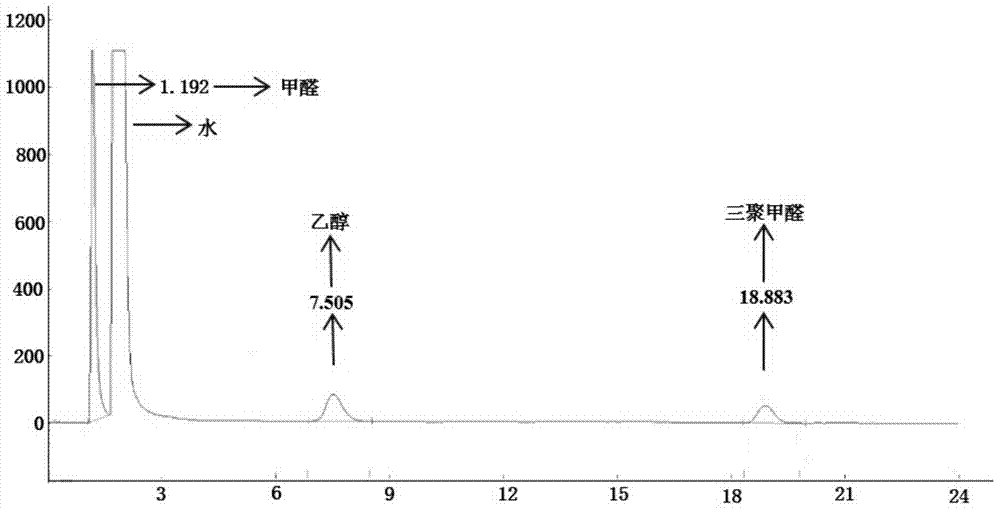
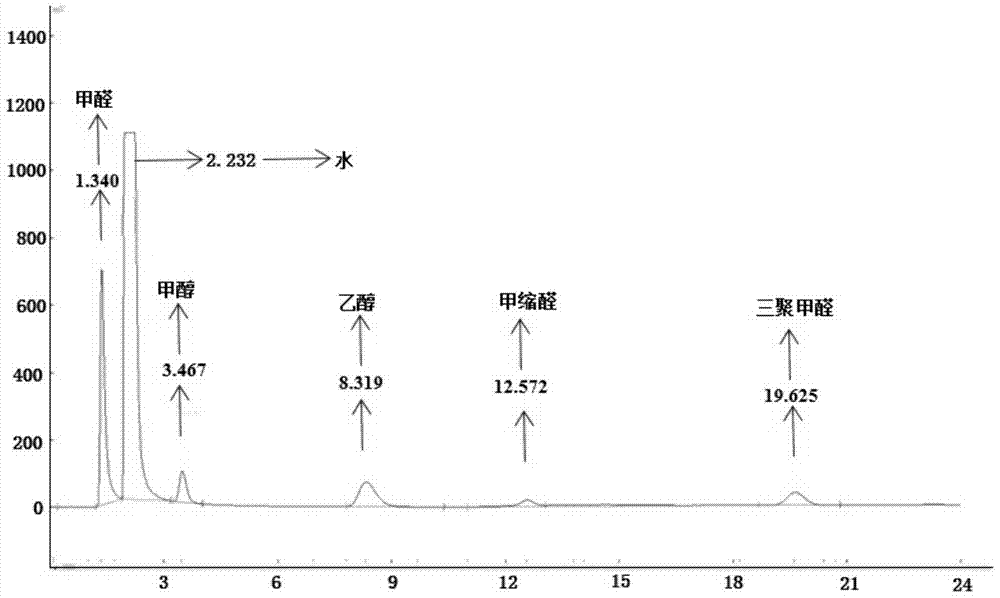
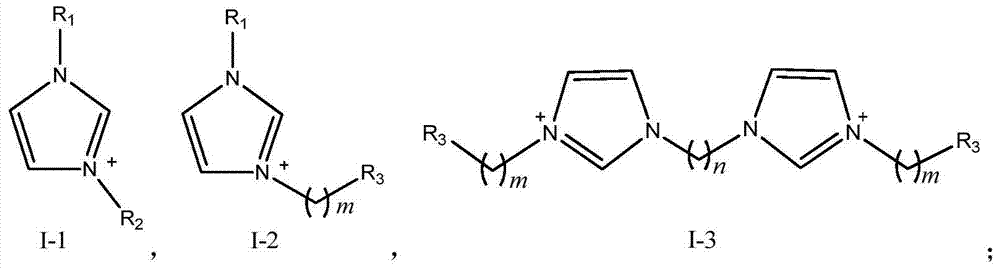
A kind of method of synthesizing trioxane
A technology of paraformaldehyde and paraformaldehyde, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve low TOX yield, high production energy consumption, latent heat of vaporization advanced questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] This embodiment provides a method for synthesizing paraformaldehyde, using ionic liquid as a solvent and catalyst to synthesize paraformaldehyde from solid paraformaldehyde, the specific steps are as follows:
[0073] Preparation of ionic liquid a: Add an appropriate amount of N-methylimidazole to a three-necked flask, slowly add equimolar 1,3-propane sultone dropwise, stir and react for 24 hours, filter with suction to obtain a white precipitate, wash with acetone, vacuum Dry to obtain the intermediate; then add the intermediate to a three-necked flask, dropwise add equimolar concentrated sulfuric acid, use ethyl acetate as a solvent, heat up to 70°C, stir and reflux for 48 hours, then wash with ethyl acetate, and dry in vacuo to obtain ions liquid a.
[0074] Preparation of paraformaldehyde: Add 6.0 g of paraformaldehyde and 30.2 g of ionic liquid a into a 50 mL reactor in sequence, control the reaction temperature at 90° C., and react for 25 minutes to obtain parafor...
Embodiment 2
[0082] This embodiment provides a method for synthesizing paraformaldehyde, using ionic liquid as a solvent and catalyst to synthesize paraformaldehyde from solid paraformaldehyde, the specific steps are as follows:
[0083]Preparation of ionic liquid b: Weigh an appropriate amount of N-methylimidazole and add it to a three-necked flask, slowly add equimolar n-bromobutane dropwise, stir and react for 48 hours, then filter with suction to obtain a white precipitate (intermediate) and wash it with acetone and vacuum dried. Then add the intermediate to the three-necked flask, dropwise add equimolar concentrated sulfuric acid, use ethyl acetate as solvent, heat up to 70°C, stir and reflux for 48 hours, then wash with ethyl acetate, and vacuum dry to obtain ionic liquid b.
[0084] Preparation of paraformaldehyde: add 4.0 g of paraformaldehyde and 30.1 g of ionic liquid b into a 50 mL reaction kettle in sequence, control the reaction temperature at 90° C., and react for 25 minutes ...
Embodiment 3
[0086] This embodiment provides a method for synthesizing paraformaldehyde, using ionic liquid as a solvent and catalyst to synthesize paraformaldehyde from solid paraformaldehyde, the specific steps are as follows:
[0087] Preparation of ionic liquid c: Add an appropriate amount of N-methylimidazole to a three-necked flask, slowly add concentrated sulfuric acid dropwise at a molar ratio of 1:1 under stirring conditions, then gradually raise the temperature to 80°C, react for 3 hours, and reduce the temperature at 0.1MPa at 70°C Distilled under pressure for 0.5h, washed with ethyl acetate, and dried in vacuo to obtain ionic liquid c.
[0088] Preparation of paraformaldehyde: sequentially add 6.1g of solid paraformaldehyde and 31.6g of ionic liquid c into a 50mL reactor, control the reaction temperature at 90°C, and react for 45min to obtain paraformaldehyde; use chromatography to analyze the product in the reactor The concentration of paraformaldehyde was found to be 6.9%, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com