Preparation method of actinomycetes fibrinolytic enzyme
A technology of fibrinolytic enzymes and actinomycetes, applied in the biological field, can solve the problems of reduced fibrinolytic enzyme activity, reduced fibrinolytic effect, and many separation and purification steps, so as to protect catalytic activity and stability, reduce losses, and process The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
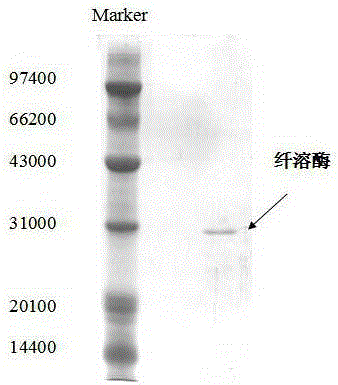
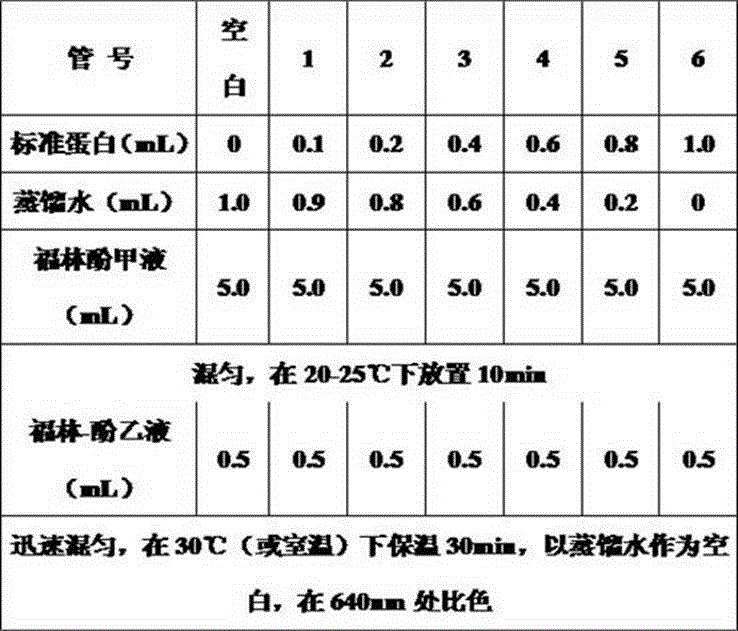
Image
Examples
Embodiment 1
[0022] 1. Landscape medium: 0.4-0.5%glucose, 0.3-0.6%yeast paste, 0.5-1.5%malt extract, 0.1-0.2%of calcium carbonate, 1.5%agar, pH6.7.
[0023] Crowding conditions: 4-7D cultivation at 28 ℃.
[0024] Seed medium: 0.4-0.5%glucose, 0.3-0.6%yeast paste, 0.5-1.5%malt extract, 0.1-0.2%of calcium carbonate, adjustment pH to 6.7, sterilization after sterilization.
[0025] Cultivation conditions: The speed is 170-190r / min, and 28 ° C is erected 28-32h as liquid seeds.
[0026] Fermentation medium: 6-8%of millet powder, 2-4%glucose, 0.1-0.3%calcium carbonate, 0.4-0.6%sodium chloride, 0.6-0.7%protein, pH6.7, 5%inoculation amount.
[0027] Cultivation conditions: 150-170r / min, 22 ° C fermentation 90-100h.
[0028] The pine bacteria YY21 liquid fermentation product is 10000R / min 20min under 4 ° C, obtained the upper liquid, and the ostelase ratio is 12.01mg / ml.
[0029] Preparation of picked bacteria fibrobine liquid: Add ammonium sulfate powder to the fermented liquid containing moltencease...
Embodiment 2
[0031] 1. Landscape training with the same embodiment 1
[0032] 2. Seed training and embodiment 1
[0033] 3. Ferment training and embodiment 1
[0034] 4. Preparation and embodiments of the compound enzyme liquid of the lines of the lines 1
[0035] 5. Octyl-sephaaroseff interaction method of hydrophobic interaction
[0036] Add ammonium sulfate powder to the crude enzyme solution to fully dissolved, so that the ammonium sulfate saturation can reach 30 %.Collect fibrous activity components and obtain a compounding ratio of 112.07mg / ml.
Embodiment 3
[0038] 1. Landscape training with the same embodiment 1
[0039] 2. Seed training and embodiment 1
[0040] 3. Ferment training and embodiment 1
[0041] 4. Preparation and embodiments of the compound enzyme liquid of the lines of the lines 1
[0042] 5. Octyl-sephaaroseff interaction layer analysis method is the same as embodiment 2
[0043] 6. Phenyl-sephaarosehp hydrophobic interaction layer analysis method
[0044] Adjust the OCTYL-sephaaroseff interaction layer to collect the ammonium sulfate of the active component to 15 %, and perform Phenyl-SEPHAROSEHP hydrophobic interaction layer.The collection of fibrous activity components is collected, and the enzyme ratio is 173.16U / mg, and the vitality recovery rate is 19.35%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com