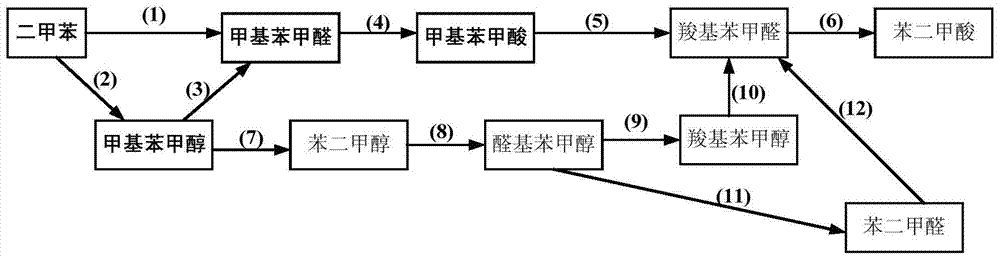
A method for co-producing methylbenzoic acid, methylbenzaldehyde and methylbenzyl alcohol
A technology of methyl benzaldehyde and methyl benzyl alcohol, applied in chemical instruments and methods, separation/purification of carboxylic acid compounds, preparation of carboxylate, etc., can solve low reaction efficiency, loss and loss of methyl benzyl alcohol, etc. problem, to achieve the effect of improving utilization rate, avoiding side reactions and improving economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
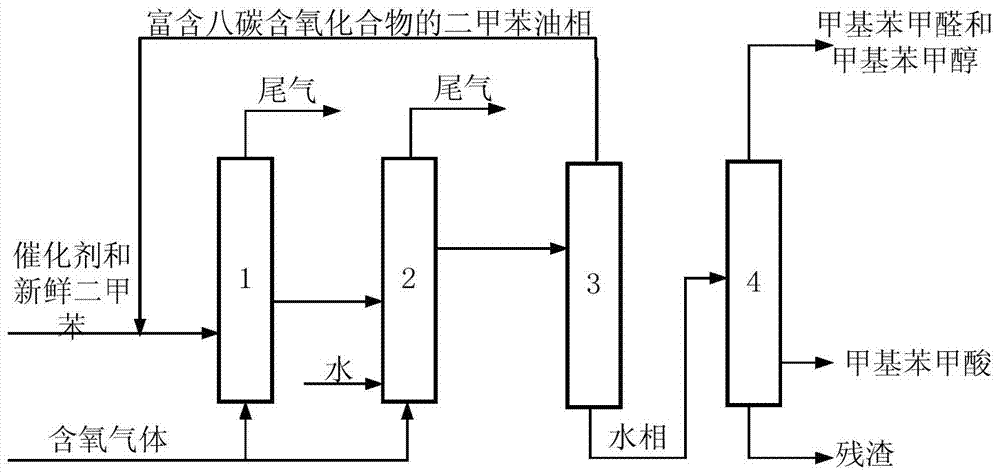
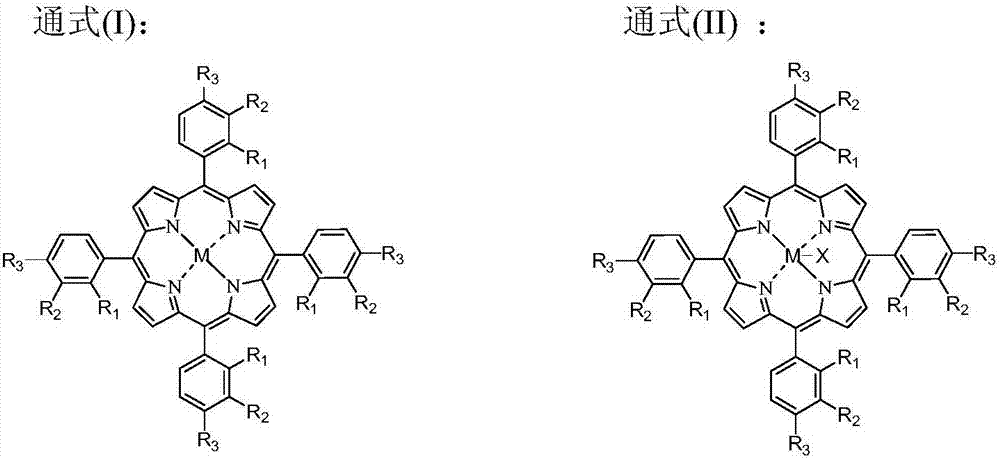
[0072] The catalyst dissolved in fresh p-xylene added to the system is HfO 2 , N-hydroxyl o-sulfonyl benzamide, metal phthalocyanine (R) with general formula (IV) structure 1 = H, R 2 =F, M=Fe) and metalloporphyrins (R 1 = R 3 =OH,R 2 =H, M=Ru) with a total concentration of 600 ppm. When the device is in steady state operation, the flow rate of fresh p-xylene added to the primary oxidation reactor is 15.7mL / h, and the flow rate of the circulating oil phase rich in eight-carbon oxygenates and p-xylene is 125.0mL / h. The total flow rate of the oxidation reactor was 140.7 mL / h. At this time, the average residence time of the primary oxidation reactor in terms of liquid phase substances is 1.6h, and the pressurized pure oxygen is continuously fed into the primary oxidation reactor to maintain the system reaction temperature at 179°C and reaction pressure at 0.6MPa. The flow rate of water added to the secondary oxidation reactor is 46.8mL / h. At this time, the volume ratio of t...
Embodiment 2
[0074] The catalyst that dissolves in the fresh p-xylene that adds in the primary oxidation reactor is cobalt naphthenate and the metal phthalocyanine (R with general formula (IV) structure 1 = H, R 2 =CH 3 CH 2 , M=Co) mixture with a total concentration of 45 ppm. When the device is running in a steady state, the flow rate of freshly reacted p-xylene added to the primary oxidation reactor is 33.8mL / h, and the flow rate of the circulating oil phase rich in eight-carbon oxygenates and p-xylene is 60.1mL / h. The total flow into the primary oxidation reactor was 93.8 mL / h. At this time, the average residence time of the primary oxidation reactor in terms of liquid phase substances is 2.4 hours, and pressurized pure oxygen is continuously fed into the primary oxidation reactor to maintain the system reaction temperature at 185°C and reaction pressure at 2.2MPa. The flow rate of water added to the secondary oxidation reactor is 84.5mL / h. At this time, the volume ratio of the amo...
Embodiment 3
[0076] The catalyst dissolved in fresh p-xylene added to the primary oxidation reactor is Mn(Ac) 2 4H 2 O, metal phthalocyanine (R) with general formula (IV) structure 1 = NO 2 , R 2 =H, M=Co), the metalloporphyrin (R 1 = R 3 = H, R 2 =CH 3 CH 2 , M=Cu) mixture with a total concentration of 120ppm. When the device is running in a steady state, the flow rate of freshly reacted p-xylene added to the primary oxidation reactor is 43.8mL / h, and the flow rate of the circulating oil phase rich in eight-carbon oxygenates and p-xylene is 106.3mL / h. The total flow into the primary oxidation reactor was 150.1 mL / h. At this time, the average residence time of the primary oxidation reactor in terms of liquid phase substances is 1.5h, and the pressurized pure oxygen is continuously fed into the primary oxidation reactor to maintain the system reaction temperature at 175°C and reaction pressure at 0.7MPa. The flow rate of water added to the secondary oxidation reactor is 42.0mL / h. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com