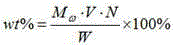Method used for preparing methylethyl ketone peroxide by taking acidic ion exchange resin as catalyst
The technology of ion exchange resin and methyl ethyl ketone peroxide is applied in the field of preparation of methyl ethyl ketone peroxide, which can solve the problems of low active oxygen content in the product, high cost of waste acid treatment, serious corrosion of strong acid, etc. high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
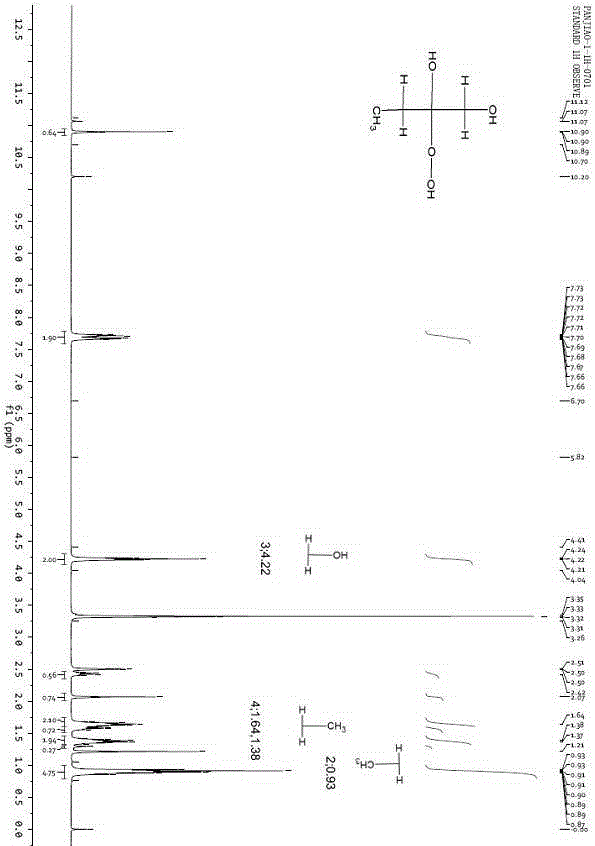
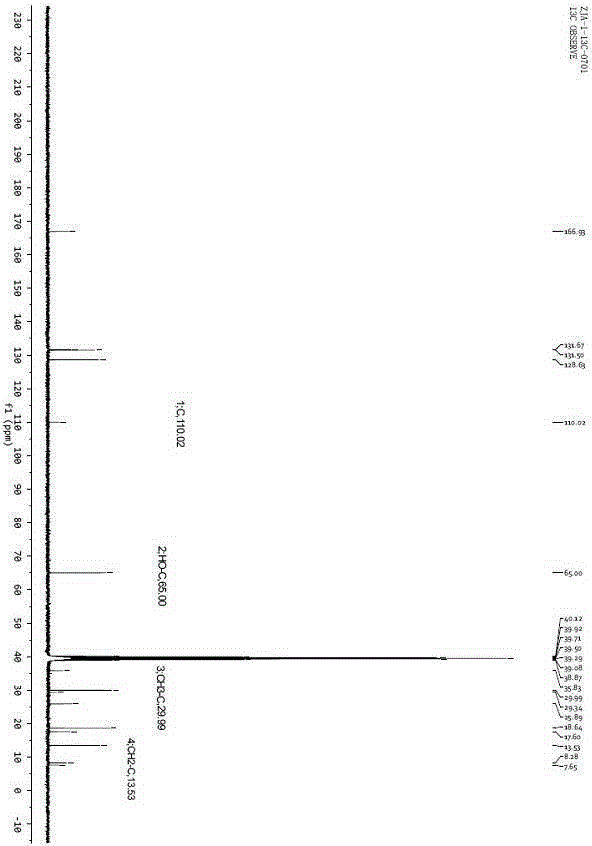
[0023] Example 1. At room temperature, add 5.67g of 30wt% hydrogen peroxide, 0.2g of Amberlyst-15 and 4.6g of dibutyl phthalate into a two-necked flask, stir and mix evenly, and adjust the temperature to about 20°C; slowly drop Add 3.6g butanone, react for 40min, then transfer to the separation funnel and let stand for 30min, separate the water phase and the oil phase, the oil phase is MEKP, the mass is 7.9g. The content of active oxygen in the oil phase was determined by chemical titration method to be 10.73%.
Embodiment 2
[0024] Example 2. At room temperature, add 5.67g of 30wt% hydrogen peroxide, 0.2g of Amberlyst-15 and 4.6g of dibutyl phthalate into a two-necked flask, stir and mix evenly, adjust the temperature to about 30°C, and slowly Add 3.6g butanone dropwise, react for 40min, then transfer to a separating funnel, let stand for 30min, separate the water phase and the oil phase, the oil phase is MEKP with a mass of 7.3g. The content of active oxygen in the oil phase was determined by chemical titration method to be 11.15%.
Embodiment 3
[0025] Example 3. At room temperature, add 5.67g of 30wt% hydrogen peroxide, 0.2g of Amberlyst-15 and 4.6g of dibutyl phthalate into a two-necked flask, stir and mix evenly, adjust the temperature to 30°C, and drop slowly during stirring Add 3.6g butanone, react for 60min, then transfer to a separating funnel, let it stand for 30min, separate the water phase and the oil phase, the oil phase is MEKP, the mass is 7.9g. The active oxygen content in the oil phase was determined to be 11.41% by chemical titration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com