Pyrene organic material, preparing method and application of pyrene organic material
A technology of organic materials and pyrenes, which is applied in the field of organic semiconductor materials, can solve the problems of reducing luminous efficiency, red shift of emission spectrum, temperature rise of devices, etc., and achieves the effects of simple synthesis method, high solubility and good flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
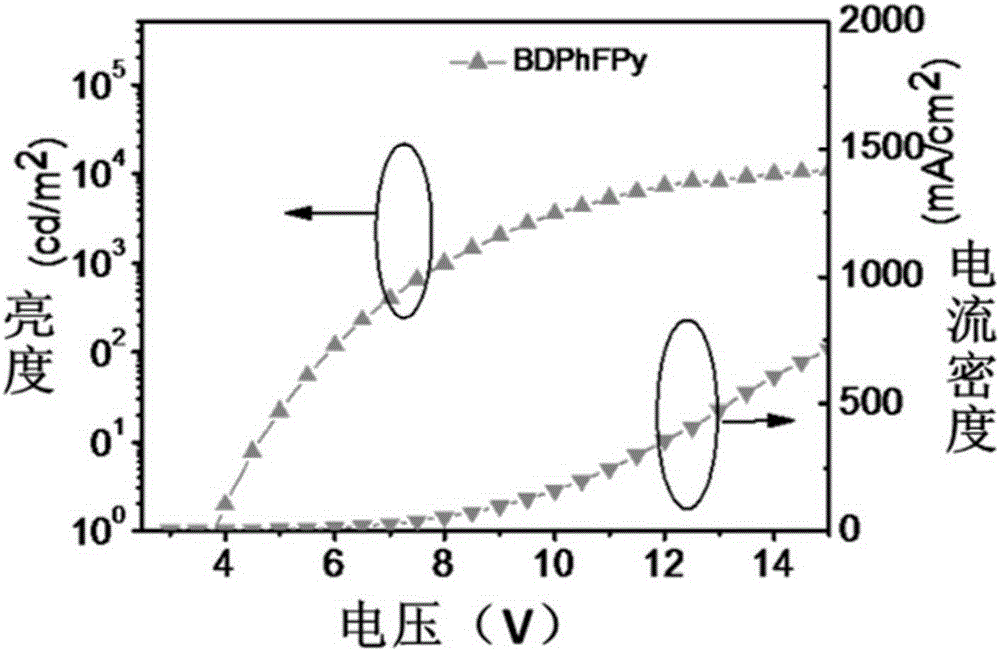
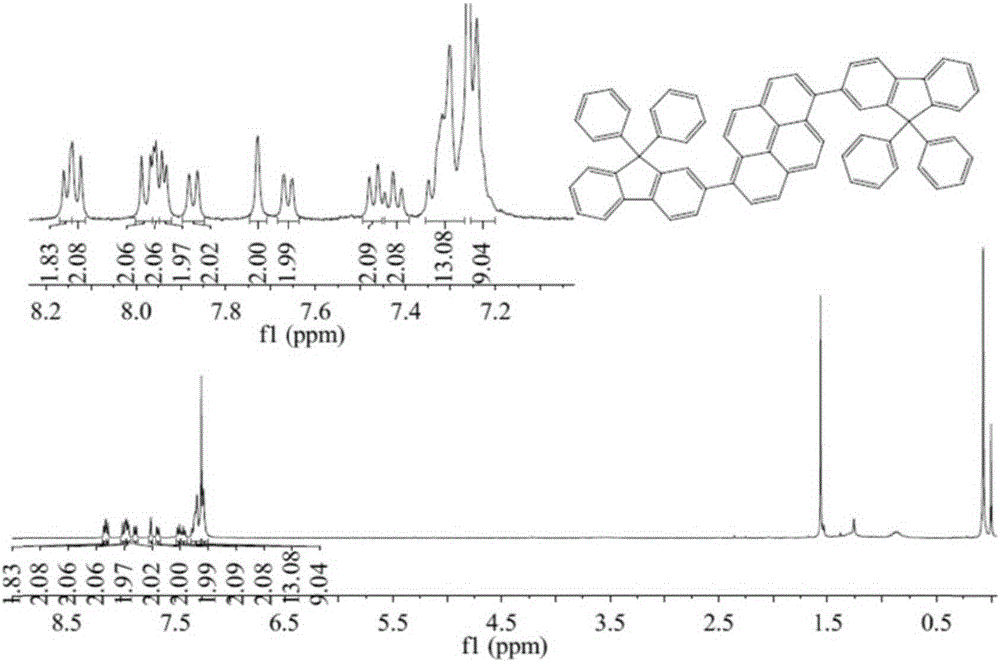
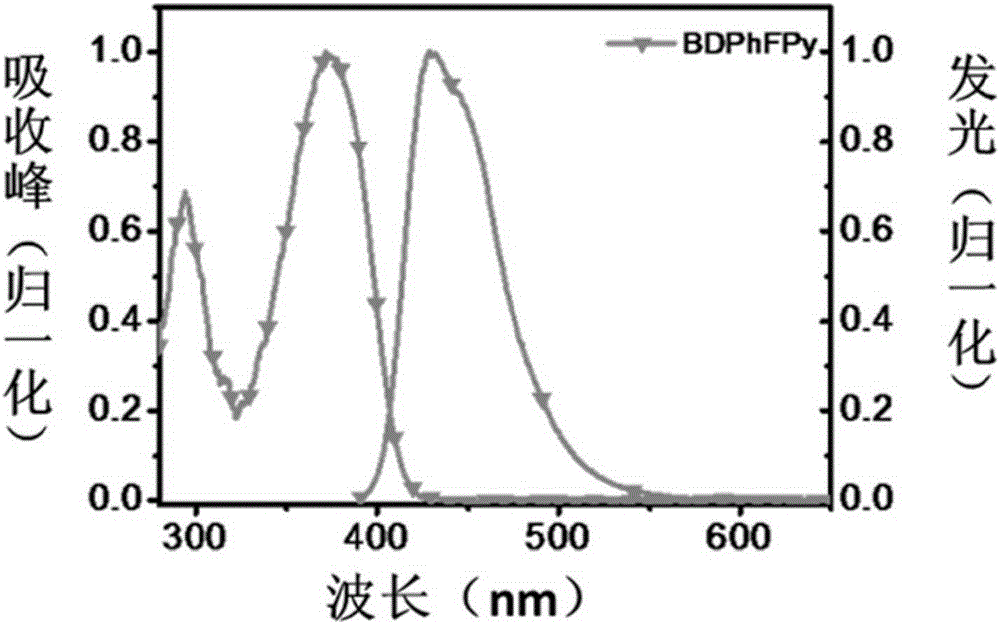
[0084] The first embodiment: the name of the pyrene-based organic material is: 1.6-bis(9,9-diphenylfluorenyl)pyrene, the chemical name is: BDPhFPy, and its chemical formula structure is as follows:
[0085]
[0086] The preparation method of diphenylfluoreneboronic acid pinacol ester is as follows:
[0087] Among them, the chemical structure of 2-bromo-9,9-diphenylfluorene is as follows:
[0088]
[0089] The chemical structure of pinacol diboronate is as follows:
[0090]
[0091] The chemical structure of 1,1'-bis(diphenylphosphino)ferrocene (DPPF) is as follows:
[0092]
[0093] The chemical structure of 1,4-dioxane is as follows:
[0094]
[0095] Accurately weighed 2-bromo-9,9-diphenylfluorene (3.9mmol), biboronic acid pinacol ester (5.9mmol), palladium acetate (Pd(OAC) 2 , 0.15mmol), potassium acetate (KOAc, 9.4mmol) and 1,1'-bis(diphenylphosphino)ferrocene (DPPF) (0.3mmol) were placed in a clean and dry two-necked flask (250ml ), vacuuming and nitrog...
no. 2 example
[0112] Second embodiment: the name of the pyrene-based organic material is: 1.6-bispirobisfluorene pyrene, the chemical name is: DSBFPy, and its chemical formula structure is as follows:
[0113]
[0114] The preparation method of spirobifluorene boronic acid pinacol ester is as follows:
[0115] Accurately weighed 2-bromo-spirobifluorene (3.9mmol), biboronic acid pinacol ester (5.9mmol), palladium acetate (Pd(OAC) 2 , 0.15mmol), potassium acetate (KOAC, 9.4mmol) and 1,1'-bis(diphenylphosphino)ferrocene (DPPF) (0.3mmol) were placed in a clean and dry two-necked flask (250ml ), vacuuming and nitrogen filling were repeated three times. Deoxygenate 1,4-dioxane for about 1 hour in advance, and draw about 40ml into the reaction bottle with a syringe. During the whole reaction process, avoid light, pass condensed water, and reflux at 90°C for 12h. After the reaction was completed, an appropriate amount of water was added to the reaction solution, the liquid was extracted and s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com