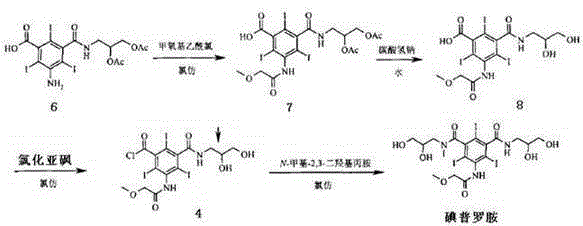
Preparation technology of iopromide intermediate
A technology of iopromide and preparation process, applied in the field of drug synthesis, can solve problems such as inability and high yield, and achieve the effects of low cost, high yield and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis of embodiment 1.5-nitroisophthalic acid dimethyl ester
[0034] In a 100mL round-bottomed flask equipped with a condenser, an electric stirrer and a thermometer, add 5.0g of 5-nitroisophthalic acid, add 33.3ml of methanol, and 2 Stir at room temperature, about 3min, the solid dissolves completely, the solution is colorless, clear and transparent, then slowly add 1.0ml of concentrated sulfuric acid under stirring, heat up and reflux, about 3h there is white solid precipitation, TLC traces the reaction, developing agent (CH 2 Cl 2 :CH 3 OH=10:1), cooled and crystallized, filtered, washed with a small amount of water, and the filter cake was dried to obtain dimethyl 5-nitroisophthalate, which was weighed and the yield was 98%. 1HNMR (CDCl 3 ) δ: 8.92-9.18 (m, 3H), 3.98 (s, 6H).
Embodiment 2
[0035] The synthesis of embodiment 2.5-nitroisophthalic acid monomethyl ester
[0036] Add 2.5g of dimethyl 5-nitroisophthalate and 38.5mL of methanol into a 250mL four-neck flask with a stirrer, condenser and thermometer, heat and stir to dissolve, then add 0.42g dropwise within 20min and dissolve with 5g of water Sodium hydroxide solution, heated and refluxed for 2h, TLC tracked the reaction until the raw material point disappeared, developing agent (petroleum ether: CH 2 Cl 2 =5:1); after taking a small amount of acid for acidification, use 5-nitroisophthalic acid and 5-nitroisophthalic acid monomethyl ester as a control, and see if there is any product formation and 5-nitroisophthalic acid on TLC. The appearance of phthalic acid, developing agent (CH 2 Cl 2 :CH 3 OH=5:1). After the reaction, methanol was distilled off, 26mL of water was added, filtered while hot, and the filtrate was acidified with concentrated hydrochloric acid to a pH value of 1, and crystals were p...
Embodiment 3
[0037] Example 3. Synthesis of 3-(2,3-dihydroxypropylcarbamoyl)-5-nitroisophthalic acid (compound 10a)
[0038] In a 50mL round-bottomed flask equipped with a condenser, an electric stirrer and a thermometer, add 400mg of 5-nitroisophthalic acid monomethyl ester, add 4ml of tert-butanol, stir at room temperature, the solid is not dissolved, add 194mg of 3-amino-1,2 -Propylene glycol and 598mg potassium tert-butoxide, the solid is dissolved, and the color of the solution changes from white to brown. Under an atmosphere of nitrogen, the temperature is raised to reflux, and the reaction is tracked by TLC until the raw material point disappears. The operation is as follows: take 1 drop of the solution, add 1 drop h 2 O, adjust the pH to 1 with concentrated HCl and spot the plate with the raw material (developing agent CH 3 OH:CH 2 Cl 2 : glacial acetic acid=2:1: glacial acetic acid), after the reaction, distill tert-butanol, add 2mlH 2 O, adjust the pH to 1 with concentrated H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com