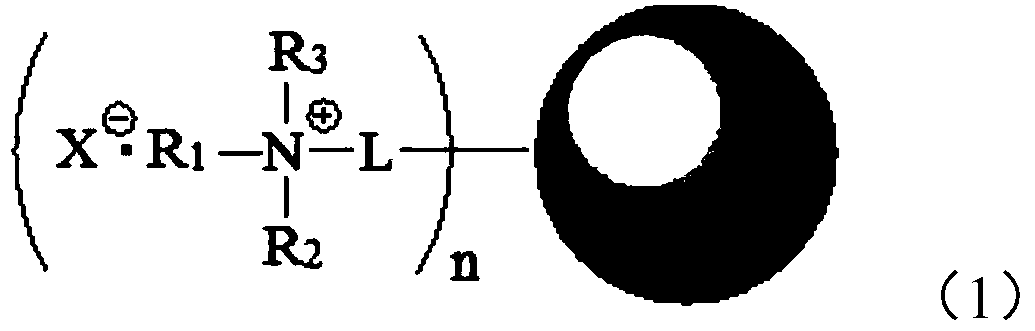
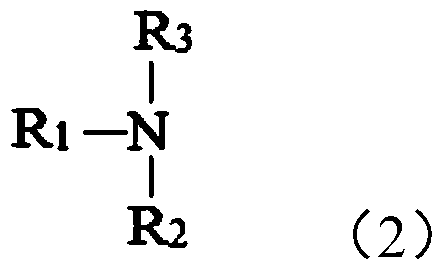
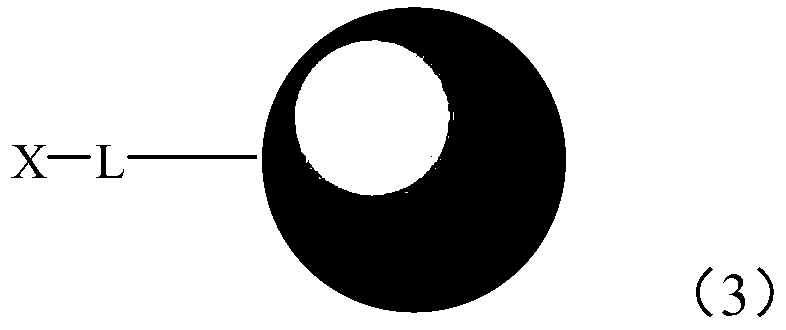
Supported quaternary ammonium catalyst, its preparation method and application
A catalyst, supported technology, applied in the preparation of oxygen-containing compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of poor catalyst stability and easy loss of active components.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Magnetic Fe 3 O 4 Preparation of nanoparticles: 14.12g Fe(acac) 3 Dissolved in a mixed solution composed of 200 mL of oleylamine and 200 mL of dibenzyl ether, the solution was treated at 110 °C for 1 hour under nitrogen protection, and then continued to be treated at 300 °C for 2 hours under nitrogen protection, and 100 mL of ethanol was added. The solid was washed three times with acetone, 100 mL each time, and the obtained solid was dried in a vacuum oven to obtain magnetic Fe 3 O 4 Nanoparticle A1 was characterized by transmission electron microscope and found that the average particle size of the nanoparticle was 9.5 nm.
[0103] Treatment of chlorosilicates: 10.0 g of magnetic Fe 3 O 4 Nanoparticle A1 was placed in a 500mL three-neck flask, and then 200mL anhydrous toluene, 2.0g γ-chloropropyl triethoxysilane (γ-chloropropyl triethoxysilane, CPTES, C 3 H 6 ClSi(OC 2 H 5 ) 3 ), refluxed at 110 °C for 24 hours, filtered, washed with absolute ethanol for 3 t...
Embodiment 2
[0107] Magnetic Fe 3 O 4 Preparation of nanoparticles: 14.12g Fe(acac) 3 Dissolved in a mixed solution composed of 400 mL of oleylamine and 50 mL of dibenzyl ether, the solution was treated at 80 °C for 5 hours under nitrogen protection, and then continued to be treated at 350 °C for 4 hours under nitrogen protection, and 100 mL of ethanol was added. The solid was washed three times with acetone, 100 mL each time, and the obtained solid was dried in a vacuum oven to obtain magnetic Fe 3 O 4 Nanoparticle A2 was characterized by transmission electron microscope and found that the average particle size of the nanoparticle was 5.9 nm.
[0108] Treatment of chlorosilicates: 10.0 g of magnetic Fe 3 O 4 Nanoparticle A2 was placed in a 500mL three-neck flask, and then 200mL anhydrous toluene, 0.2g γ-chloropropyl triethoxysilane (γ-chloropropyl triethoxysilane, CPTES, C 3 H 6 ClSi(OC 2 H 5 ) 3), filtered after refluxing for 6 hours, washed 3 times with absolute ethanol, and d...
Embodiment 3
[0112] Magnetic Fe 3 O 4 The preparation method of nanoparticles A2 is the same as [Example 2], and the process of chlorosilicate treatment is the same as that of [Example 1], except that the chlorosilicate used is CPTMS: 3-chloropropyl trimethoxysilane (3-chloropropyl trimethoxysilane) trimethoxysilane), the structural formula is ClC 3 H 6 Si(OCH 3 ) 3 , the carrier B3 was obtained. After weighing, it was found that B3 contained chlorosilicate and magnetic Fe. 3 O 4 The weight ratio of nanoparticles was 0.069.
[0113] The quaternization process is the same as the quaternization process described in [Example 1], except that the tertiary amine used is tributylamine, and the obtained Cl - Type-loaded quaternary ammonium salt C3.
[0114] Cl - The transformation of the supported quaternary ammonium salt is the same as the process described in [Example 1] to obtain catalyst D3, wherein the weight content of the quaternary ammonium salt component is 11.2%, and the rest is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com