Double-target anti-HIV (anti-human immunodeficiency virus) glycopeptide compound and application thereof
A compound and oligosaccharide technology, which is applied in the direction of peptides, decapeptides, antiviral agents, etc., can solve the problems of high cost, long time, and low success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, the synthesis of mannopentaose
[0021]
[0022] Weigh compound 1 (prepared according to the document J.Carbohydr.Chem., 2006, 25, 491-498, 3.67mmol) in a 100ml reaction bottle, add 10ml of dichloromethane solvent, and add 40ml of MeOH solvent to dissolve the raw material, 40 ℃ oil Heating in a bath, adding excess AcCl to carry out selective deprotection reaction, concentrating the reaction solution, and passing through a silica gel column for separation and purification to obtain compound 2 with a yield of 85%. 1 HNMR (500MHz, CDCl 3 ): δ5.98(t, J=9.9Hz, 1H, H-4), 5.63(dd, 1H, H-3), 5.46(s, 1H, H-1), 4.79-4.77(m, 1H, H-5),4.60(dd,J 6a,5 =2.3Hz,J 6a,6b =12.05Hz,1H,H-6a),4.54(dd,J 6b,5 =5.55Hz,J 6b,6a =12.05Hz,1H,H-6b),4.42(s,1H,H-2),2.77-2.65(m,2H,SC H 2 ), 1.34(t, J=7.35Hz, C H 3 );+cESI-MSforC 29 h 28 o 8 S + ,[M] + , Calcd: 536.150, Found: 536.148.
[0023]
[0024] Weigh compound 2 (0.128mmol) in the reaction flask, equipped with a...
Embodiment 2
[0029] Embodiment 2, the synthesis of sugar chain
[0030]
[0031] Under nitrogen protection, compound 7 or 8 (obtained according to the literature J.Org.Chem., 2005,70,9809-9813) or 9 (according to the literature BeilsteinJ.Org.Chem., 2010,6,801-809) or 10 (according to Literature Biomacromolecules, 2012,13,3039-3045 obtained) (0.224mmol) and compound 15 (obtained according to literature J.Med.Chem., 2015,58,1372-1379, 1.0~2.0eq) mixture was dissolved in 2 / 1 In tetrahydrofuran / water (4.5ml in total), under stirring at room temperature, add copper sulfate pentahydrate (the amount of copper sulfate pentahydrate added accounts for 5% of the molar weight of compound 7 or 8 or 9 or 10), then add sodium ascorbate (ascorbic acid The amount of sodium added accounted for 5% of the molar amount of compound 7 or 8 or 9 or 10), and transferred to 35°C oil bath reaction, after 18h, TLC (developing solvent was ethyl acetate / methanol / water of 5 / 2 / 1.5) It was found that the reaction of ...
Embodiment 3
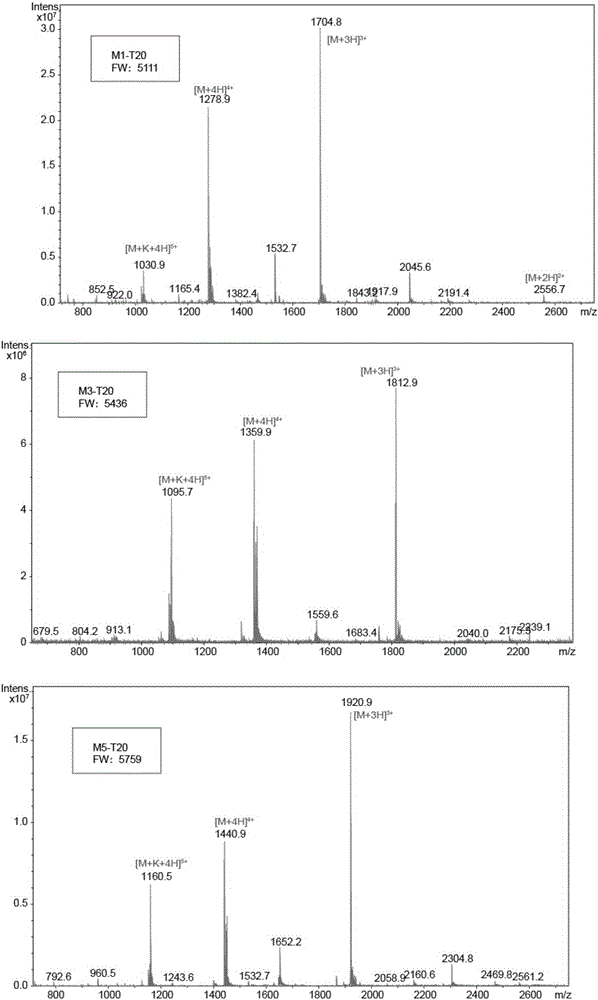
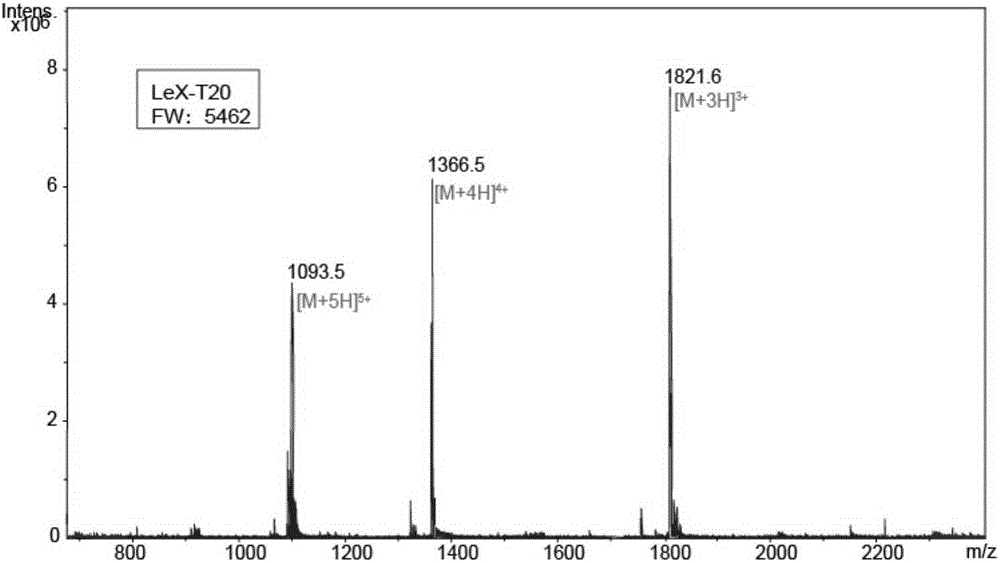
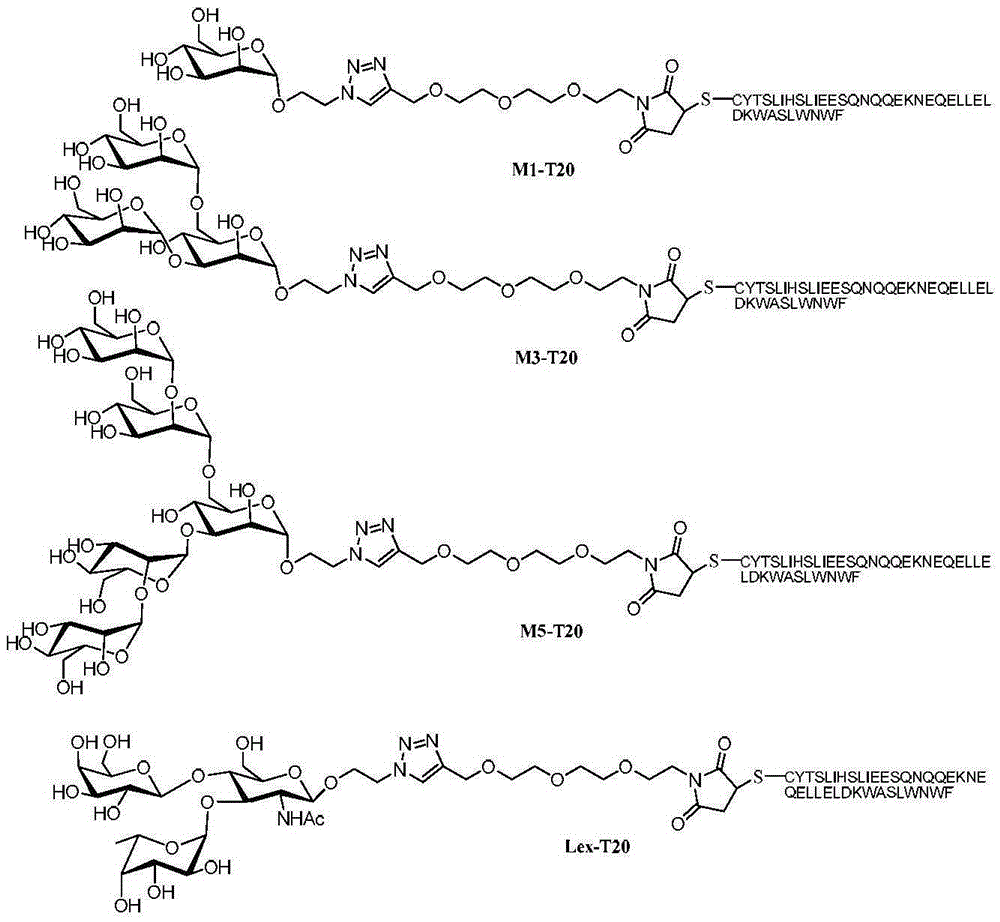
[0036] Example 3, Glycosylation modification of polypeptide (that is, synthesis of M1-T20, M3-T20, M5-T20 and Lex-T20)
[0037] The amino acid sequence of the T20 polypeptide is shown in sequence 1 in the sequence listing. Polypeptide cT20 was purchased from Polypeptide Synthesis Company, and its amino acid sequence is shown in sequence 2 in the sequence listing. The polypeptide cT20 is obtained by adding a cysteine before the nitrogen-terminal amino acid tyrosine of the T20 polypeptide, and the newly added cysteine residue serves as the site of glycosylation reaction.
[0038] The sugar chains 11-14 prepared above were connected to the nitrogen-terminal amino acid cysteine of the polypeptide cT20 through a highly chemoselective Michael addition reaction, and the specific process was as follows.
[0039] Dissolve the mixture of 0.006 mmol of sugar chains 11 to 14 obtained in Example 2 and 0.002 mmol of polypeptide cT20 in 5 ml of sodium dihydrogen phosphate / disodium hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com