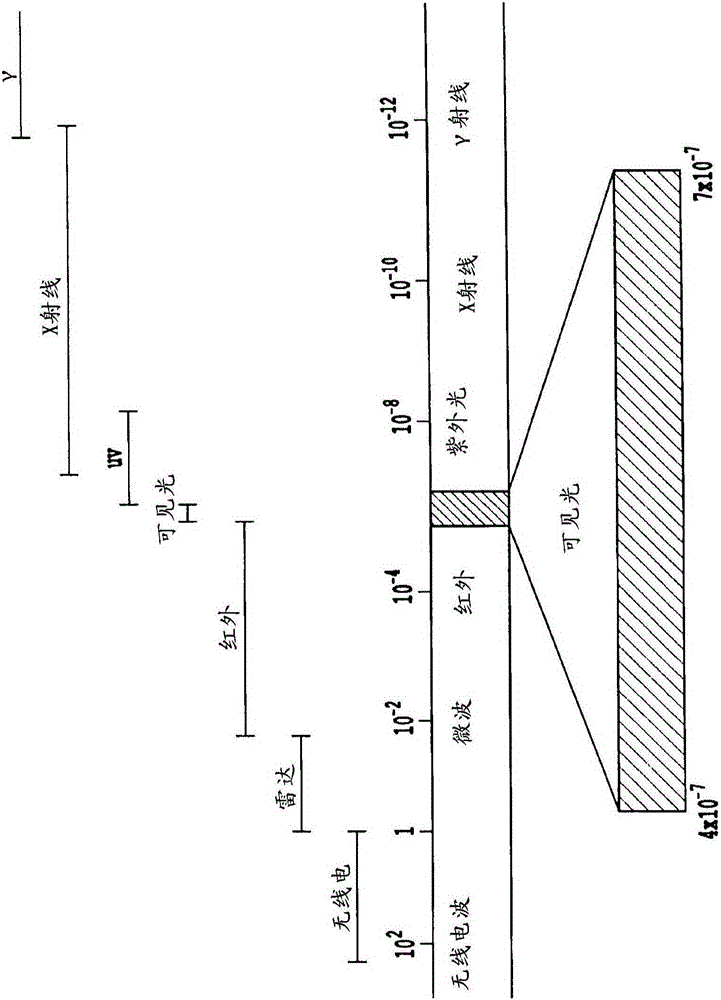
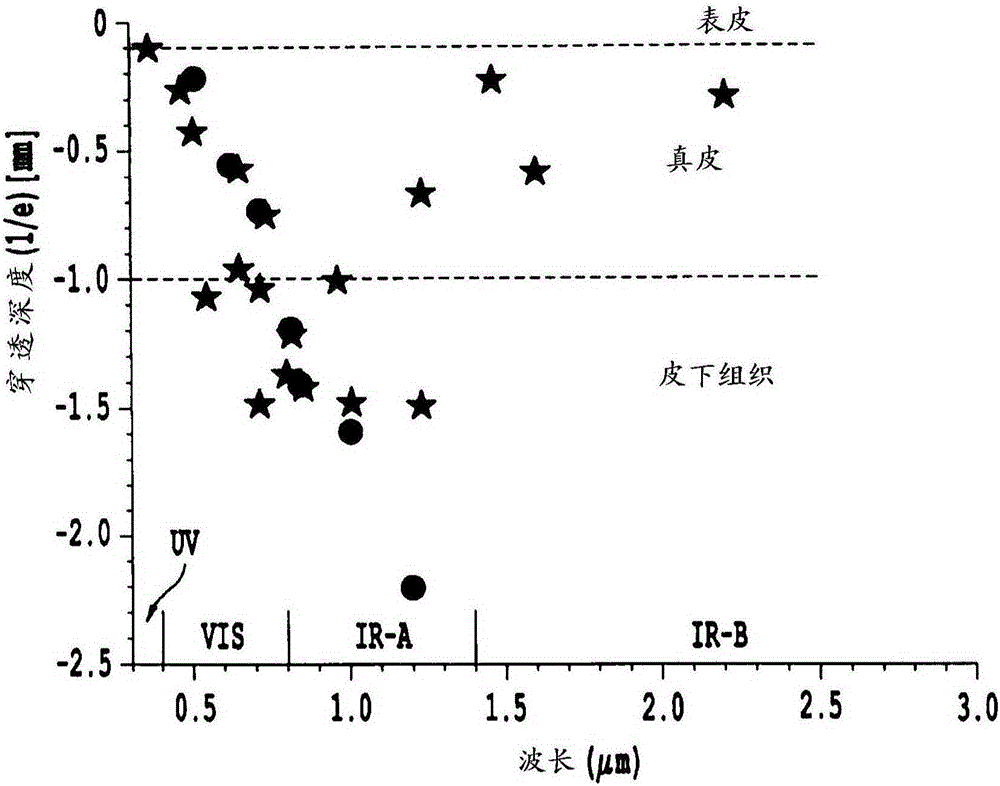
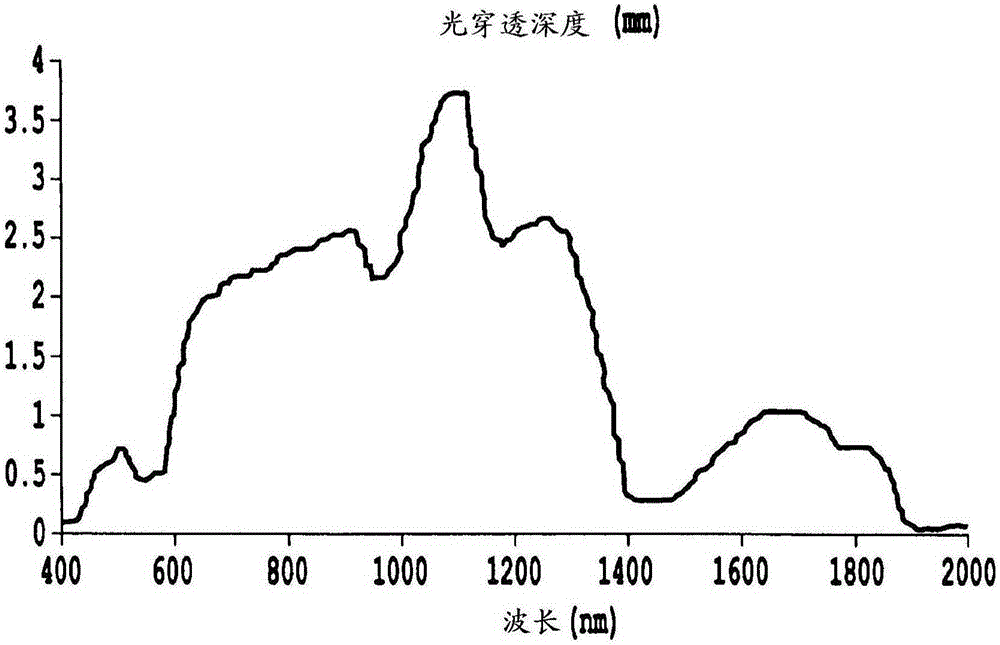
Non-invasive systems and methods for in-situ photobiomodulation
一种生物活性、调节剂的技术,应用在光疗法、消化系统、光动力疗法等方向,能够解决治疗依赖等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0564] Preparation of liposomes. The liposome preparation method was modified from Wait P., Bach, M., T., Nahde, T., Hoffmann, S., Müller, R., and Kontermann, R.E., Novel RG Dlipopeptides for the targeting of liposomes to integrin-expressing endothelia and melanoma cells. Protein Engineering Design and Selection, 2004.17(5): p.433-441]. Briefly, lipid PEG-DPPE, PC and Rh-DPPE were mixed in chloroform in a round bottom flask and evaporated (Hieroglyph Rotary Evaporator, Rose Scientific Ltd., Edmonton, Alberta, Canada) to remove chloroform. The dry film was dehydrated into the aqueous phase using PBS solution. Dry lipid films were prepared by rotary evaporation from a mixture of PC, cholesterol and PEG-DPPE, then hydrated into the aqueous phase using PBS. The mixture was mixed vigorously by vortexing, then the suspension was extruded through a polycarbonate filter (Avestin Inc., Ottawa, ON, Canada) (pore size 0.8 μm) using a Liposofast apparatus in a water bath (Instrume...
Embodiment
[0648] Preparation of silver nanoparticles
[0649] Silver (or gold) colloids were prepared according to the standard Lee-Meisel method: 200ml10 -3 M AgNO 3 The aqueous solution was boiled, then 5 ml of 35-mM sodium citrate solution was added, and the resulting mixture was kept boiling for 1 hour. It has been reported that this method yields ~10 11 Colloidal particles with a uniform size of 1 particle / ml, the diameter is ~35-50nm, and the maximum absorption is at 390nm. The colloidal solution was stored at 4°C and protected from room light. The colloidal solution was further diluted with distilled water.
[0650] Fabrication / Preparation of Metallic Nanocaps
[0651] One approach involves the use of nanospheres spin-coated on a solid support to create and control the desired roughness. The nanostructured support is subsequently covered with a silver layer, which provides the conduction electrons required for the surface plasmon mechanism. Among solid support based method...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com