Functional albumin and preparation method of nano preparation of functional albumin
A nano-functionalization technology of albumin, applied in the direction of albumin peptide, serum albumin, ovalbumin, etc., can solve the problems of unanticipated clinical effect, toxic and side effects, and interference with the pharmacokinetics of anticancer drugs, etc. Achieve the effects of simple preparation method, effective treatment, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
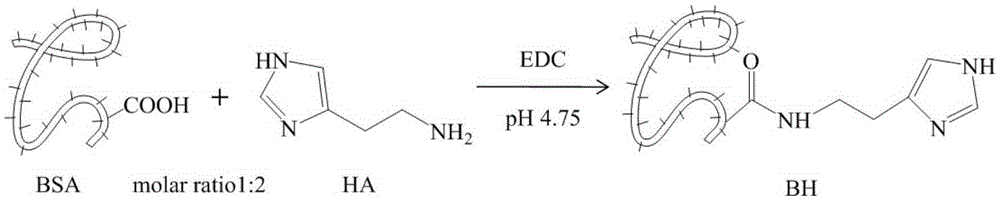
[0032] like figure 1As shown, under stirring conditions, add 13.875g of histamine dihydrochloride (HA) into 50ml of 50% (W / V) bovine serum albumin (BSA) neutral phosphate buffer solution, and then use 1M HCl solution to adjust When the pH value of the system reached 4.75, 4.5 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) was finally added for catalysis, and the reaction was stirred at room temperature for 6 hours. After the reaction was terminated by adding 4M acetate buffer salt, a dialysis bag with a molecular weight cut-off of 14,000 was used for dialysis in deionized water for 3 days; filtered, frozen at -80°C, and dried to obtain imidazolized albumin (BH).
Embodiment 2
[0034] Under stirring conditions, add 17.2g of agmatine sulfate (Agm) into 50ml of 50% (W / V) human serum albumin (HSA) neutral phosphate buffer solution, and then use 1M HCl solution to adjust the pH value of the system To 4.75, finally add 4.5g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) to catalyze, and stir at room temperature for 6h. After the reaction was terminated by adding 4M acetate buffer salt, a dialysis bag with a molecular weight cut-off of 14,000 was used for dialysis in deionized water for 3 days; filtered, frozen at -80°C, and dried to obtain guanidinated albumin (HSA-Agm).
Embodiment 3
[0036] Under stirring conditions, add 15.252g of spermine (SPE) into 50ml of 50% (W / V) human serum albumin (HSA) neutral phosphate buffer solution, and then use 1M HCl solution to adjust the pH value of the system to 4.75, Finally, 4.5 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) was added for catalysis, and the reaction was stirred at room temperature for 6 h. After the reaction was terminated by adding 4M acetate buffer salt, a dialysis bag with a molecular weight cut-off of 14,000 was used for dialysis in deionized water for 3 days; filtered, frozen at -80°C, and dried to obtain guanidinated albumin (HSA-SPE).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com