Injectable composite material for bone repair and preparation method thereof
A composite material and bone repair technology, applied in the fields of material science, orthopedics and plastic surgery, can solve the problems of complex extraction process, single component, immune and inflammatory response, etc., achieve excellent biocompatibility, enhance biological activity, induce bone effect of growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
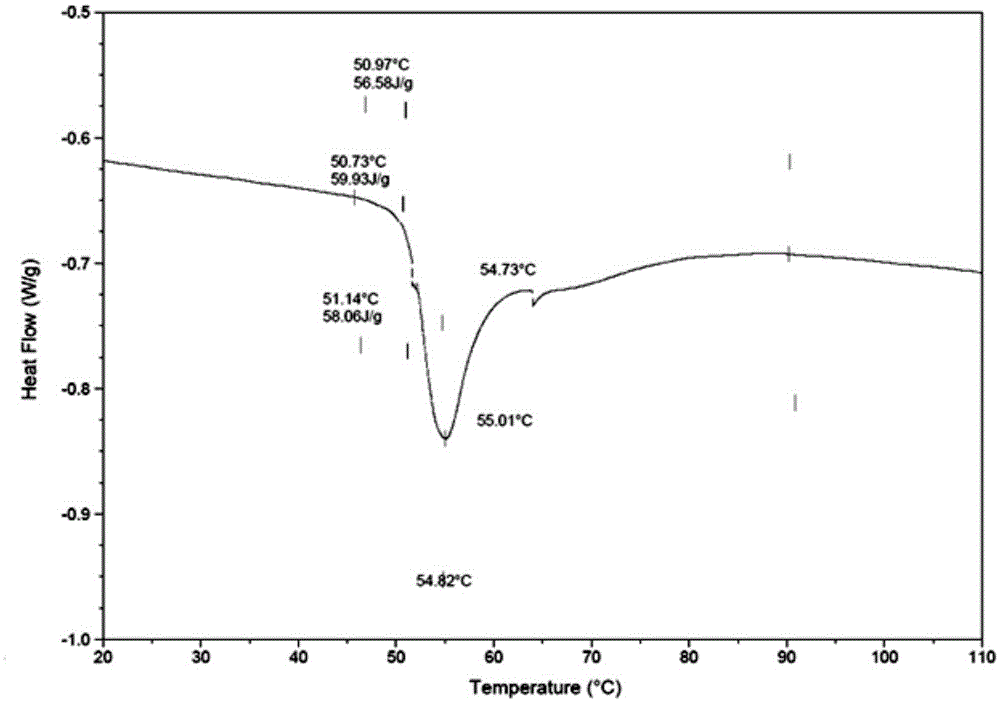
[0059] Example 1: Preparation and Characterization of Biological Tissue Matrix / Calcium Hydrogen Phosphate Composite Material
[0060] 1) Take the microfibrous biological tissue matrix material after Co60 gamma ray-sterilized pig skin decellularization and antigen removal, and suspend it in 0.9% physiological saline at a concentration of 10 mg / ml;
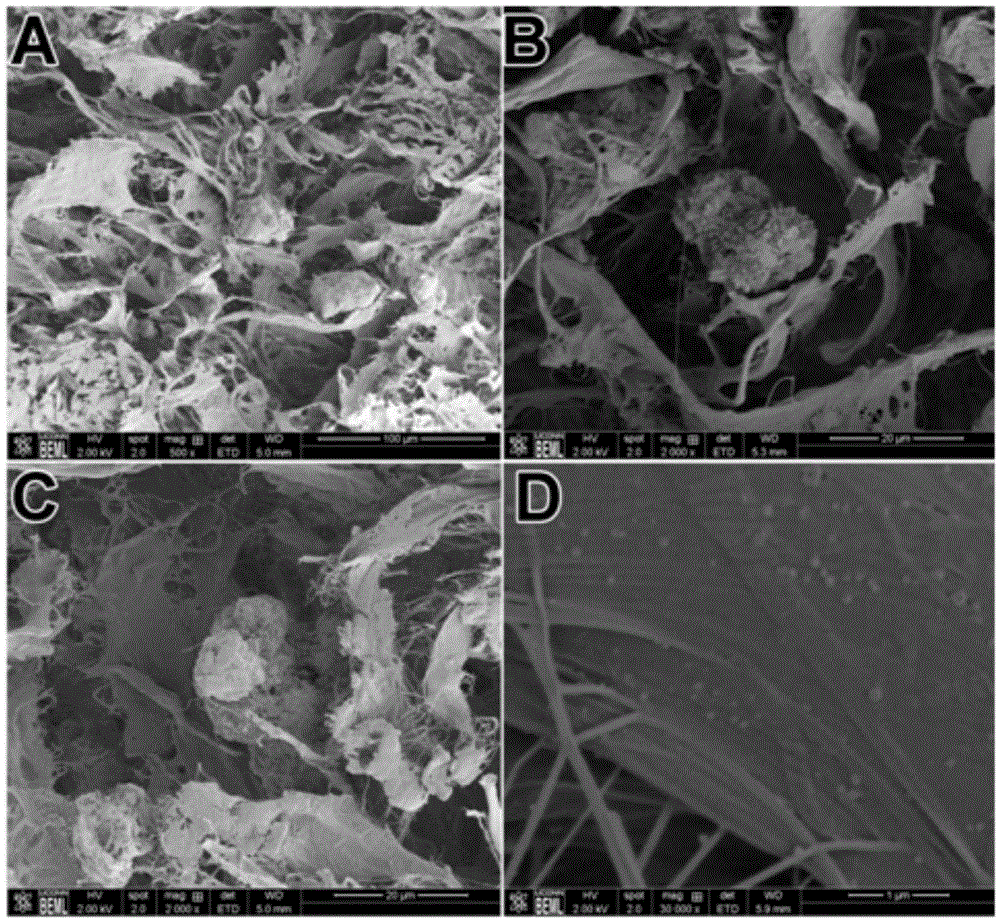
[0061] 2) Wash more than three times with sterile deionized water, the parameters of the prepared acellular matrix microfibers are as follows: figure 1 As shown, its average aspect ratio is 0.05-0.95, and its particle size distribution is 40-1000 microns;
[0062] 3) Take calcium hydrogen phosphate microparticles, add sterile deionized water, and prepare a uniform suspension of 10 mg / ml
[0063] 4) Dibasic calcium phosphate microparticles are rapidly passed through a filter membrane with a particle diameter of 40 microns to remove excessively large agglomerated particles;
[0064] 5) Centrifuge the suspension at 1200 rpm for 2 min...
Embodiment 2
[0072] Example 2: Bone defect repair experiment of biological tissue matrix / calcium hydrogen phosphate composite injectable material
[0073] The injectable biological tissue matrix / calcium hydrogen phosphate composite material prepared by the invention is used to evaluate the bone defect repairing ability of the material in the skull defect model of athymic nude mice. After the mouse (6 examples) was anesthetized, the head fur was shaved off, and a 1 cm opening was opened on the top of the head. After the skin was pulled away, a prototype defect with a diameter of 3.5 mm was punched on the skull with a dental drill; the material prepared by the present invention After the bone defect was filled, the wound was sutured; six weeks later, the mice were humanely sacrificed, and the repaired skull was observed with the naked eye, and the material and the surrounding bone tissue were well compounded, and X-ray observations showed that a large amount of bone tissue was formed in the d...
Embodiment 3
[0074] Example 3: Bone defect repair experiment of biological tissue matrix / calcium hydrogen phosphate composite injectable material as fresh bone marrow carrier
[0075] The injectable biological tissue matrix / calcium hydrogen phosphate composite material prepared in Example 1 was mixed with freshly extracted mouse whole bone marrow to prepare a pasty material; using the mouse model used in Example 2, the mixed mouse whole bone marrow The hybrid composite material was used to fill the bone defect, and the wound was then sutured; six weeks later, X-ray observations revealed that the entire prototype bone defect had been filled with newly formed bone tissue, and no tissue gaps were found at the edge of the bone defect. Micro-CT scan atlases showed the same result, the entire bone defect had been filled with newly formed bone tissue, and a seamless connection was formed between the new bone and the autogenous bone. Quantitative analysis of bone formation shows that the material ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com