Ambroxol salbutamol solution
A technology of ambroxol salbutamol and salbutamol sulfate, which is applied in the field of pharmaceutical preparations, can solve problems such as easy production of by-products, non-durable effect, and reduced effect, and achieve the goal of treating acute and chronic bronchitis, increasing the content of active ingredients, and improving stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
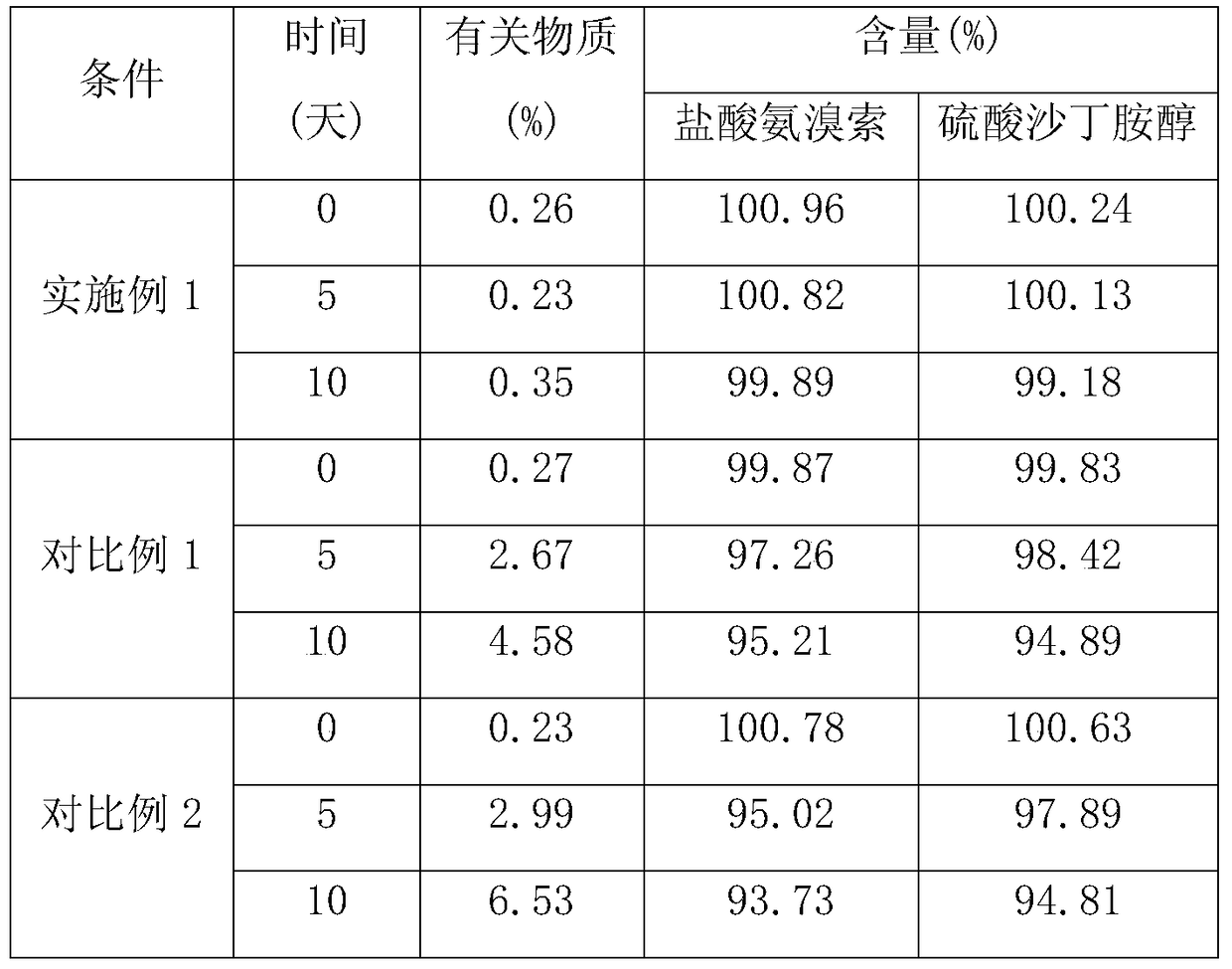
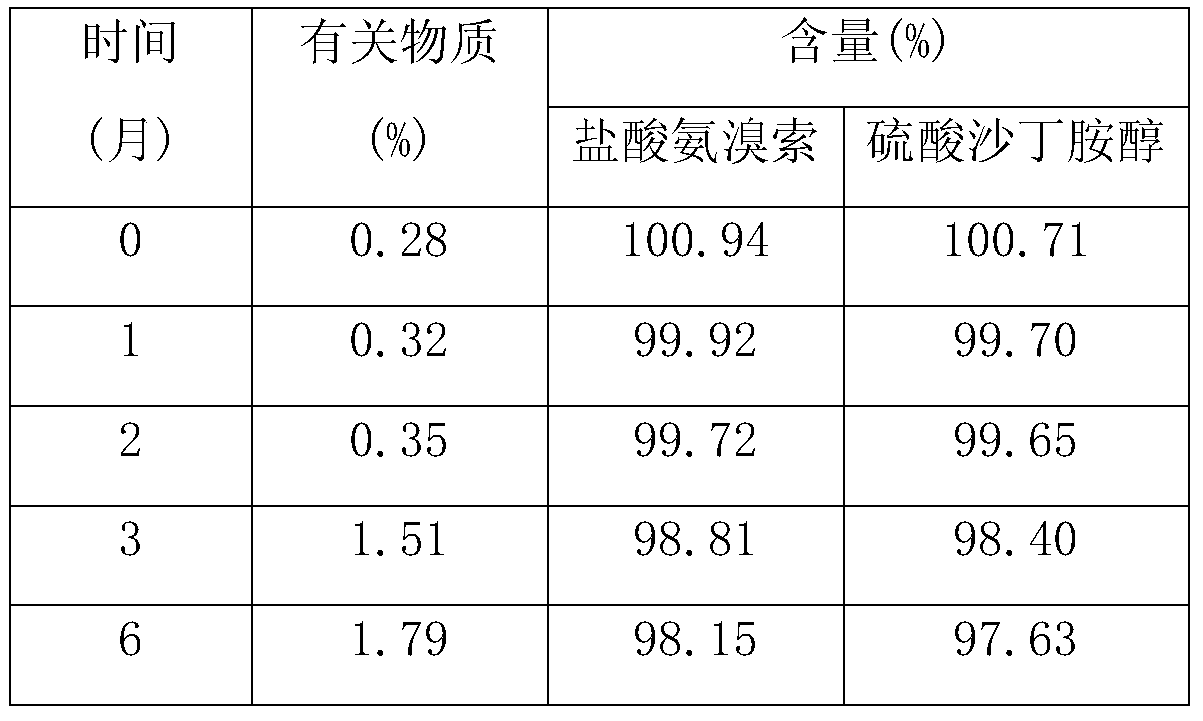
Embodiment 1
[0041] prescription:
[0042] Salbutamol sulfate: 10g;
[0043] Ambroxol hydrochloride: 20g;
[0044] Poloxamer 188:8g;
[0045] Meglumine: 8g;
[0046] Mannitol: 4g;
[0047] Propylene glycol: 15g;
[0048] Methylparaben: 1g;
[0049] Stevioside: 8g;
[0050] Purified water: add to 5L.
[0051] Its preparation method is:
[0052] 1) take by weighing the purified water of recipe quantity 80%, add the ambroxol hydrochloride of recipe quantity, stir and make it dissolve completely, continue to add the salbutamol sulfate, poloxamer 188, meglumine, mannitol and propylene glycol of recipe quantity, 50 Stir at ℃ for 20min until all dissolved;
[0053] 2) Add purified water to the full amount, add methylparaben and stevioside, stir to dissolve, filter with a 0.45 μm microporous membrane, fill, seal, pass the inspection, and package.
Embodiment 2
[0055] prescription:
[0056] Salbutamol sulfate: 10g;
[0057] Ambroxol hydrochloride: 20g;
[0058] Poloxamer 188:10g;
[0059] Meglumine: 8g;
[0060] Mannitol: 4g;
[0061] Propylene glycol: 15g;
[0062] Methylparaben: 0.5g;
[0063] Sorbitol: 8g;
[0064] Purified water: add to 5L.
[0065] Its preparation method is:
[0066] 1) take by weighing the purified water of recipe quantity 70%, add the ambroxol hydrochloride of recipe quantity, stir and make it dissolve completely, continue to add the salbutamol sulfate, poloxamer 188, meglumine, mannitol and propylene glycol of recipe quantity, 50 Stir at ℃ for 20min until all dissolved;
[0067] 2) Add purified water to the full amount, add methylparaben and sorbitol, stir to dissolve, filter with a 0.45 μm microporous membrane, fill, seal, pass the inspection, and package.
Embodiment 3
[0069] prescription:
[0070] Salbutamol sulfate: 8g;
[0071] Ambroxol hydrochloride: 15g;
[0072] Poloxamer 188:8g;
[0073] Meglumine: 6g;
[0074] Mannitol: 2g;
[0075] Propylene glycol: 10g;
[0076] Methylparaben: 0.5g;
[0077] Sucrose: 6g;
[0078] Purified water: add to 5L.
[0079] Its preparation method is:
[0080] 1) take by weighing the purified water of recipe quantity 60%, add the ambroxol hydrochloride of recipe quantity, stir and make it dissolve completely, continue to add the salbutamol sulfate, poloxamer 188, meglumine, mannitol and propylene glycol of recipe quantity, 55 Stir at ℃ for 25min until all dissolved;
[0081] 2) Add purified water to the full amount, add methylparaben and sucrose, stir to dissolve, filter with a 0.45 μm microporous membrane, fill, seal, pass the inspection, and package.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com