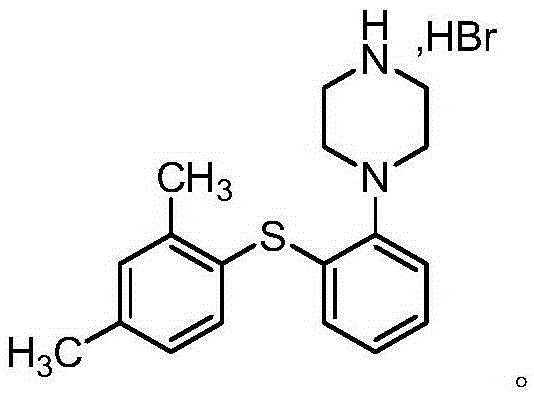
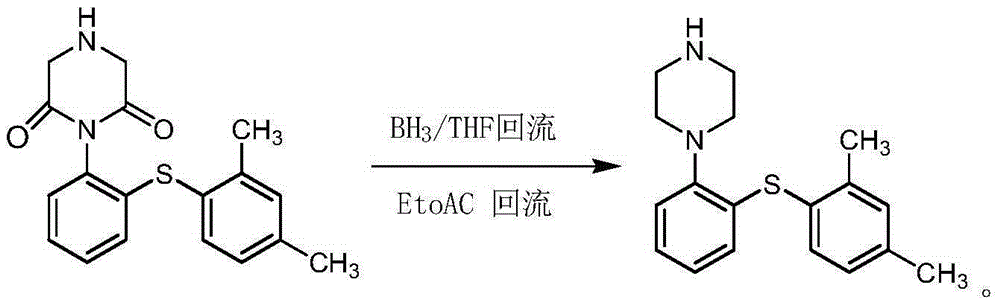
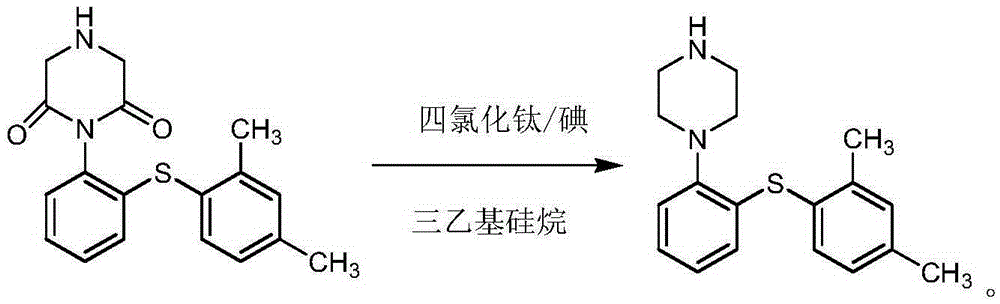
Preparation method of Vortioxetine
A vortioxetine, dripping technology, applied in the field of preparation of vortioxetine, can solve the problems of high toxicity of borane, high reaction temperature, unsuitable for large-scale production, etc., to improve production efficiency, high yield, Ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 100 g of 1-[2-(2,4-dimethyl-phenylthio)-phenyl]-piperazine-2,6-dione (compound 1) was dissolved in 500 ml of ethyl acetate. Add 500ml of ethyl acetate into a 2L reaction flask, cool down to 0-10°C, add 72.7g of titanium tetrachloride and 2.5g of iodine (protected under nitrogen), slowly add 178.1g of triethylsilane dropwise, and complete the dropwise addition in 15 minutes . Then slowly add the prepared Compound 1 solution dropwise, the dropwise addition is completed in 45 minutes, and the temperature is controlled at 0-10°C; after the dropwise addition, the temperature is raised to 25-30°C, and the reaction is stirred for 5h. Slowly drop the reaction system into 200ml of water to quench, and control the temperature at 0-10°C. After the dropwise addition, separate the organic phase, wash the organic phase with saturated sodium chloride solution for 3 times, and then dry over anhydrous sodium sulfate , concentrating under reduced pressure and drying to obtain 87.8 g of ...
Embodiment 2
[0029] 100 g of 1-[2-(2,4-dimethyl-phenylthio)-phenyl]-piperazine-2,6-dione (compound 1) was dissolved in 500 ml of dichloromethane. Add 500ml of dichloromethane into a 2L reaction flask, cool down to 0-10°C, add 75.0g of titanium tetrachloride and 2.8g of iodine (protected under nitrogen); slowly add 180.0g of triethylsilane dropwise, and complete the dropwise addition in 10 minutes . Then slowly add the prepared Compound 1 solution dropwise, and the dropwise addition is completed in 50 minutes. After the dropwise addition, the temperature is raised to 25-30° C., and the reaction is stirred for 4.5 hours. Slowly drop the reaction system into 200ml of water to quench, and control the temperature at 0-10°C. After the dropwise addition, separate the organic phase, wash the organic phase with saturated sodium chloride solution for 3 times, and then dry over anhydrous sodium sulfate , Concentrated under reduced pressure and dried, 88.5 g of white solid was obtained with a yield o...
Embodiment 3
[0031] 100 g of 1-[2-(2,4-dimethyl-phenylthio)-phenyl]-piperazine-2,6-dione (compound 1) was dissolved in 500 ml of dichloromethane. Add 500ml of dichloromethane into a 2L reaction flask, cool down to 0-10°C, add 85.0g of titanium tetrachloride and 3.5g of iodine (protected under nitrogen); slowly add 185.0g of triethylsilane dropwise, and complete the dropwise addition in 15 minutes . Then slowly add the prepared Compound 1 solution dropwise, the dropwise addition is completed in 50 minutes, and the temperature is controlled at 0-10°C; after the dropwise addition, the temperature is raised to 25-30°C, and the reaction is stirred for 5h. Slowly drop the reaction system into 200ml of water to quench, and control the temperature at 0-10°C. After the dropwise addition, separate the organic phase, wash the organic phase with saturated sodium chloride solution for 3 times, and then dry over anhydrous sodium sulfate , concentration under reduced pressure and drying, 88.8 g of white...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com