High-yield preparing method for inorganic halogen perovskite fluorescent quantum dots at room temperature
A fluorescent quantum dot, perovskite technology, applied in chemical instruments and methods, luminescent materials, nano-optics, etc., can solve problems such as increasing cost, reducing material synthesis efficiency, reducing product quality and monodispersity, and achieving excellent performance. , the effect of simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (a) Weigh 0.0556gPbCl 2 , 0.0738gPbBr 2 , 0.0269gCsCl, 0.0341gCsBr, (the molar ratio is Cs:Pb:Cl:Br=1:1:1.5:1.5) was dissolved in 10mL of DMF, and 1mL of oleic acid and 0.5mL of oleylamine were added. Because PbCl 2 It is difficult to dissolve with CsCl, so add 3mL dimethyl sulfoxide (DMSO) to aid in dissolution.
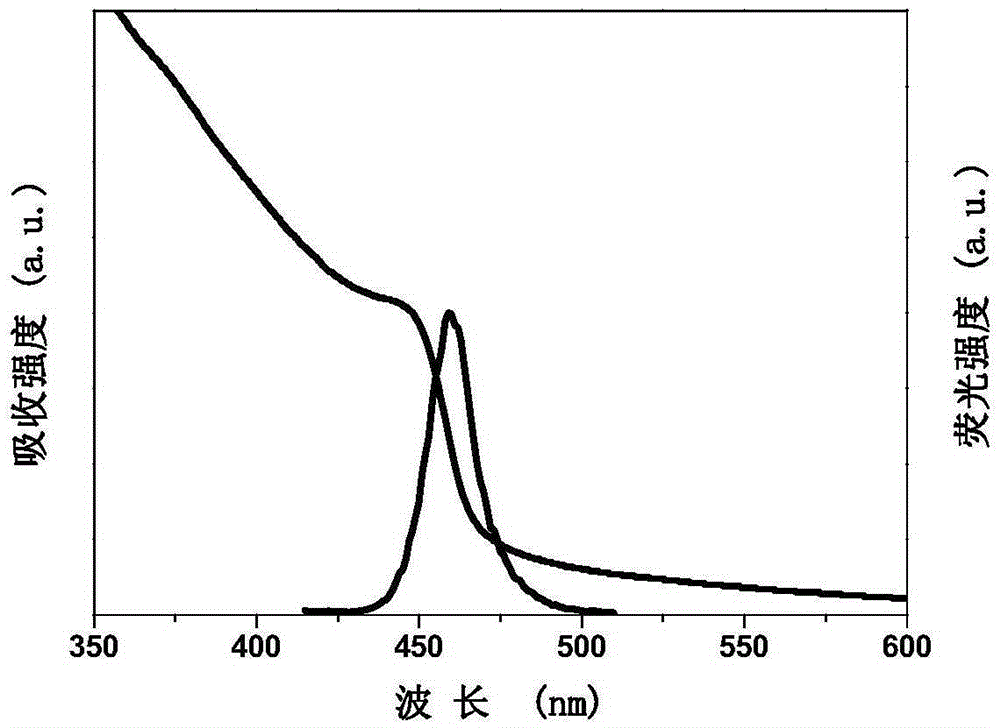
[0020] (b) After stirring and dissolving for 10 min, measure 2 mL of the precursor solution and drop it into 10 mL of toluene at a speed of 0.1 mL / s, and keep stirring at a speed of 1000 rpm. The luminescent quantum dots were formed within 5s. All operations are carried out at normal temperature and pressure without protective gas. Such as figure 1 As shown, the maximum emission wavelength of the prepared luminescent quantum dot is 455nm, the crystal structure is a perovskite structure, the full width at half maximum is 16nm, and the fluorescence quantum efficiency is 37%.
Embodiment 2
[0022] (a) Weigh 0.0371gPbCl 2 , 0.0984gPbBr 2 , 0.018gCsCl, 0.0454gCsBr, (the molar ratio is Cs:Pb:Cl:Br=1:1:1:2) was dissolved in 10mL of DMF, and 1mL of oleic acid and 0.5mL of oleylamine were added. Because PbCl 2 It is difficult to dissolve with CsCl, so add 3mL dimethyl sulfoxide (DMSO) to aid in dissolution.
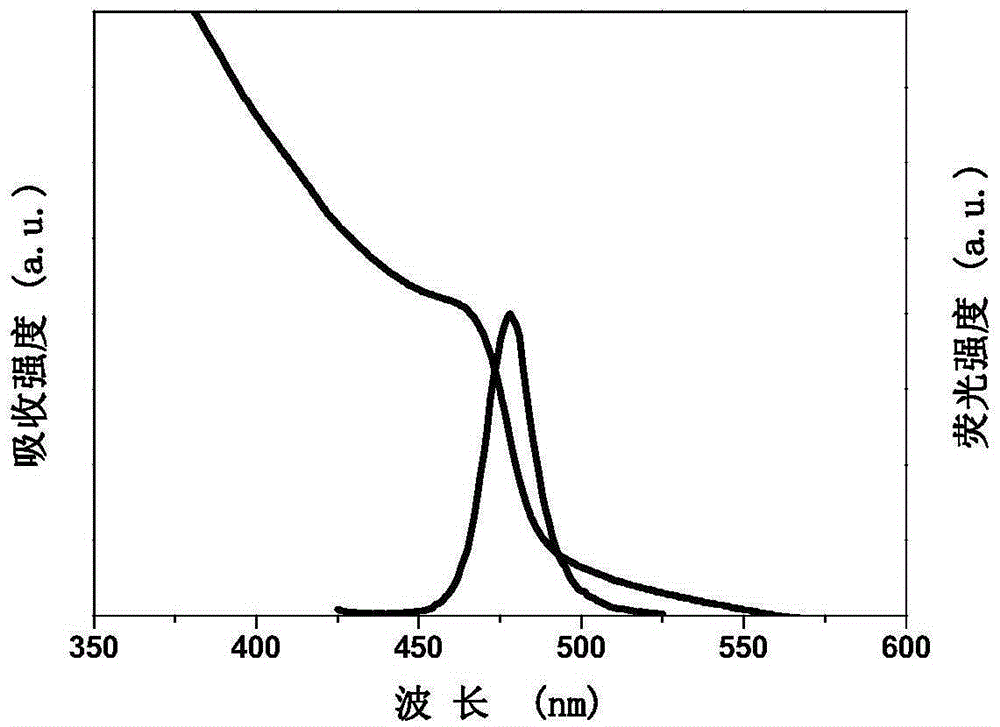
[0023] (b) After stirring and dissolving for 10 min, measure 2 mL of the precursor solution and drop it into 10 mL of toluene at a speed of 0.08 mL / s, and keep stirring at a speed of 1000 rpm. The luminescent quantum dots were formed within 5s. All operations are carried out at normal temperature and pressure without protective gas. Such as figure 2 As shown, the maximum emission wavelength of the prepared luminescent quantum dot is 478nm, the crystal structure is a perovskite structure, the full width at half maximum is 18nm, and the fluorescence quantum efficiency is 62%.
Embodiment 3
[0025] (a) Weigh 0.0681g of PbBr 2 , 0.1476g of CsBr, (the molar ratio of Cs:Pb:Br=1:1:3) was dissolved in 10mL of DMF, and 1mL of oleic acid and 0.5mL of oleylamine were added.
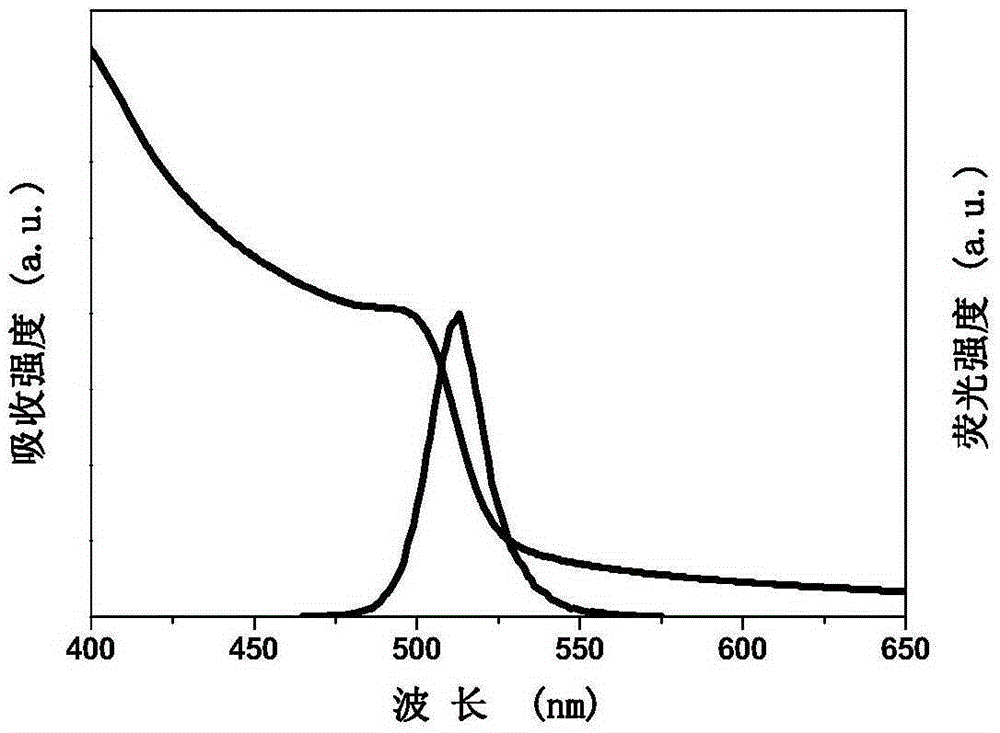
[0026] (b) After stirring and dissolving for 10 min, measure 2 mL of the precursor solution and drop it into 10 mL of toluene at a speed of 0.13 mL / s, and keep stirring at a speed of 1000 rpm. Luminescent quantum dots are formed within 1 s. All operations are carried out at normal temperature and pressure without protective gas. Such as image 3 As shown, the maximum emission wavelength of the prepared luminescent quantum dot is 513nm, the crystal structure is a perovskite structure, the full width at half maximum is 20nm, and the fluorescence quantum efficiency is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com