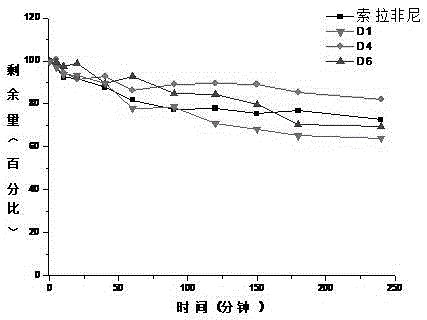
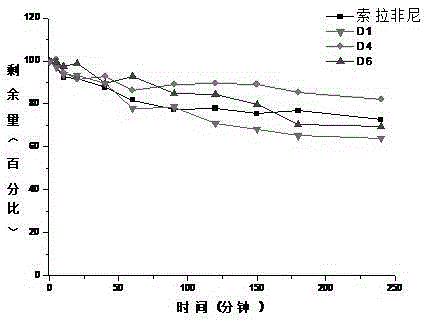
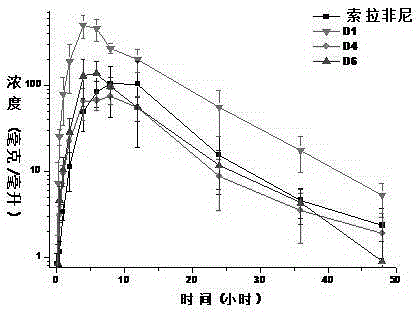
Deuterated bisarylurea compound and preparation method thereof, and application of compound in preparation of antitumor drug
A bisaryl urea and ether compound technology, which is applied in the fields of chemistry and medicine, can solve the problems of increased difficulty in continued use, complete remission and partial remission of advanced renal cancer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: The synthetic route of compound D1 is as follows:
[0049]
[0050] (1) Dissolve 3 g of 4-chloropyridine-2-carboxylic acid methyl ester in 8 ml of 40% methylamine aqueous solution by mass ratio, react at room temperature for 3 hours, after the reaction is complete, add dichloromethane for extraction, dry, filter, concentrate and evaporate to dryness The oil was obtained with a yield of 87%, which was directly used in the next reaction.
[0051] (2) Dissolve the oil in step (1) in tetrahydrofuran for later use; take 700 mg of 1,5-dideuterium-4-p-aminophenol and add it to 10 ml of N,N-dimethylformamide for dissolution, then add 815 mg of t- Potassium butoxide, then add the above-mentioned tetrahydrofuran solution in which the oil is dissolved, dropwise; heat to 100°C for reaction, after the reaction is complete, pour the reaction solution into water, extract with dichloromethane, dry, filter, concentrate and evaporate to dryness, and the column layer Analy...
Embodiment 2
[0054] Embodiment 2: The synthetic route of compound D2 is as follows:
[0055]
[0056] (1) Dissolve 206mg of methyl 4-chloropyridine-2-carboxylate in tetrahydrofuran for later use; then take 100mg of deuterated methylamine hydrochloride and add water to dissolve it, then add 242mg of triethylamine. The solution was reacted at room temperature for 3 hours. After the reaction was complete, water and dichloromethane were added for extraction. The organic layer was separated, dried, concentrated and evaporated to dryness to obtain an oil with a yield of 78%, which was directly used in the next reaction.
[0057] (2) Dissolve the oil in step (1) in tetrahydrofuran for later use, take 700mg of 1,5-dideuterium-4 aminophenol and add it to 10ml of N,N-dimethylformamide to dissolve, then add 815mg of tert-butanol Potassium, then add the above-mentioned tetrahydrofuran solution of dissolved oil dropwise, and the drop is completed; heat to 100°C for reaction, after the reaction is co...
Embodiment 3
[0060] Embodiment 3: The synthetic route of compound D3 is as follows:
[0061]
[0062] (1) Dissolve 206mg of methyl 4-chloropyridine-2-carboxylate in tetrahydrofuran for later use; add 100mg of deuterated methylamine hydrochloride into water to dissolve, then add 242mg of triethylamine, after the addition is complete, drop it into the above-mentioned tetrahydrofuran solution, React at room temperature. After the reaction is complete, add water and dichloromethane to extract, separate the organic layer, dry, concentrate and evaporate to dryness to obtain an oily substance with a yield of 78%, which is directly used in the next reaction.
[0063] (2) Dissolve the oil in step (1) in tetrahydrofuran for later use, take 1.81g of p-aminophenol and add 25ml of N,N-dimethylformamide to dissolve, then add 2.5g of potassium tert-butoxide, and then dropwise add The above tetrahydrofuran solution in which the oil was dissolved was dripped and heated to 100°C for reaction. After the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com