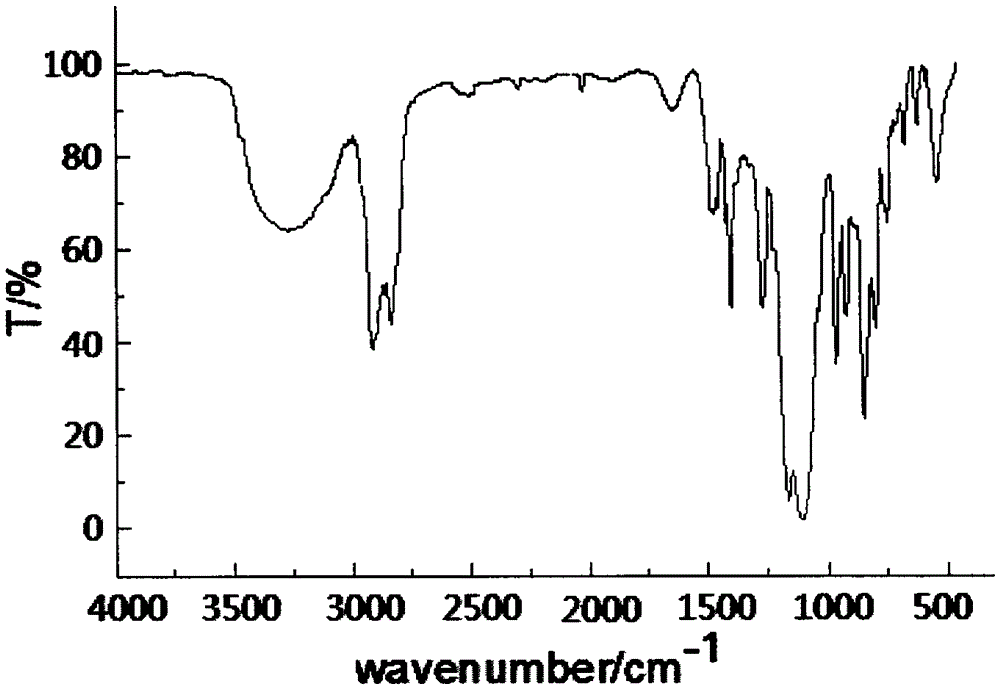
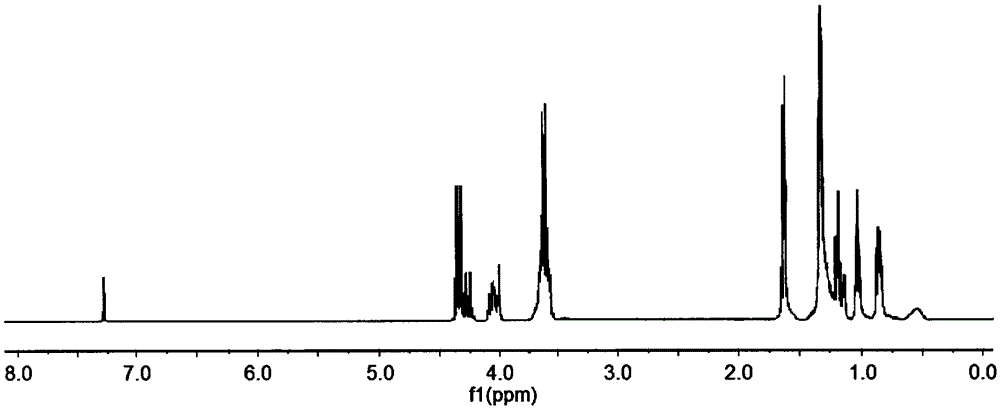
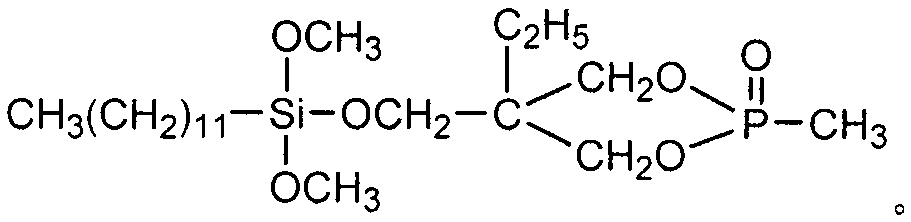Dodecyl dimethyoxy phosphonic heterocyclic methyl ester compound and preparation method thereof
A technology of dodecyl dimethoxy phosphine and heterocyclic methyl ester is applied in the field of dodecyl dimethoxy phosphine heterocyclic methyl ester compound and preparation thereof, and can solve serious pollution, limited application scope and toxicity. Large and other problems, to achieve the effect of high decomposition temperature, small mechanical properties, and no three waste emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 In a 200ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency reflux condenser, the air in the bottle was replaced with nitrogen, and 16.21g (0.10mol) of caged phosphoric acid ester and 80ml of diethylene glycol dimethyl ether were added. Solution, 29.06g (0.10mol) dodecyltrimethoxysilane and 0.45g dimethyl sulfate, heated up to 160°C, kept warm for 14h, then cooled to 60°C, transferred to a separatory funnel to stand for layering, Separate the lower layer of feed liquid, then add 62ml of toluene to wash, let stand to separate the lower layer of feed liquid, then distill under reduced pressure to remove toluene and a small amount of low boiling point matter, to obtain light yellow liquid dodecyl dimethoxyphosphine heterocyclic methyl ester, Product yield 87.5%, flash point (open cup): 206±5°C, decomposition temperature: 293±5°C, density (25°C): 1.713g / cm 3 , Refractive index: n D 25 = 1.4036.
Embodiment 2
[0027] Example 2 In a 200ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency reflux condenser, replace the air in the bottle with nitrogen, and add 16.21g (0.10mol) of cage-like phosphate, 31.97g (0.11mol) of twelve Alkyl trimethoxysilane and 0.32g dimethyl sulfate, heat up to 155°C, reflux and keep warm for 16h, then cool to 60°C, add 62ml of toluene to wash, let stand to separate the lower layer of material liquid, and then distill under reduced pressure to remove toluene and A small amount of low-boiling point substances gave light yellow liquid dodecyl dimethoxyphosphine heterocyclic methyl ester, the product yield was 86.7%, its flash point (open cup): 206±5°C, decomposition temperature: 293±5°C, Density (25°C): 1.713g / cm 3 , Refractive index: n D 25 = 1.4036.
Embodiment 3
[0028]Example 3 In a 200ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency reflux condenser, replace the air in the bottle with nitrogen, and add 16.21g (0.10mol) of caged phosphate ester, 20mlDMF, 34.87g (0.12mol) Dodecyltrimethoxysilane and 0.49g methyl p-toluenesulfonate were heated up to 130°C, kept for 19 hours, then cooled to 60°C, transferred to a separatory funnel to stand for stratification, and the lower layer of material liquid was separated. Then add 62ml of toluene to wash, let stand to separate the lower layer feed liquid, then distill under reduced pressure to remove toluene and a small amount of low boiling point, to obtain light yellow liquid dodecyl dimethoxyphosphine heterocyclic methyl ester, the product yield is 88.2%, Its flash point (open cup): 206±5°C, decomposition temperature: 293±5°C, density (25°C): 1.713g / cm 3 , Refractive index: n D 25 = 1.4036.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com