Composition containing amlodipine besylate and preparation method of composition
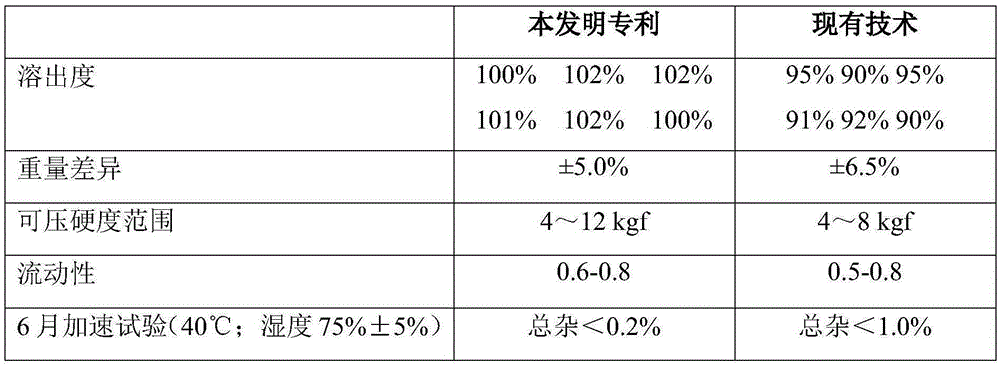
A kind of technology of amlodipine besylate and composition, applied in the field of composition containing amlodipine besylate and the field of preparation thereof, can solve problems such as unfavorable drug dissolution, small difference in tablet weight, slow tablet disintegration and the like , to achieve the effect of reducing equipment and workshop investment, improving weight difference, and fast disintegration or dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A composition containing amlodipine besylate, characterized in that: said composition comprises the following components in parts by weight:
[0034] 5 parts of amlodipine besylate; 90 parts of filler; 0.4 parts of disintegrant; 1.1 parts of lubricant.
[0035] The composition is prepared as a tablet.
[0036] The filler is microcrystalline cellulose.
[0037] The disintegrant is sodium carboxymethyl starch.
[0038] Described lubricant is magnesium stearate.
[0039] The method for preparing the described composition containing amlodipine besylate is characterized in that, the method may further comprise the steps:
[0040] 1) Ingredients: weigh each component according to the prescription quantity;
[0041] 2) Premix 1: add 3 / 4 of the filler to amlodipine besylate, mix for 3 minutes, and obtain material 1;
[0042] 3) Sieving: pass material 1 through a 14-mesh sieve to obtain material 2;
[0043] 4) Premix 2: Mix material 2 for 12 minutes to obtain material 3; ...
Embodiment 2
[0051] A composition containing amlodipine besylate, characterized in that: said composition comprises the following components in parts by weight:
[0052] 5 parts of amlodipine besylate; 195 parts of filler; 2.7 parts of disintegrant; 3.1 parts of lubricant.
[0053] The composition is prepared as a tablet.
[0054] The filler is calcium hydrogen phosphate dihydrate.
[0055] The disintegrant is croscarmellose sodium.
[0056] Described lubricant is magnesium stearate.
[0057] The method for preparing the described composition containing amlodipine besylate is characterized in that, the method may further comprise the steps:
[0058] 1) Ingredients: weigh each component according to the prescription quantity;
[0059] 2) Premix 1: add 3 / 4 filler to amlodipine besylate, mix for 6 minutes, and obtain material 1;
[0060] 3) Sieving: pass material 1 through a 18-mesh sieve to obtain material 2;
[0061] 4) Premix 2: Mix material 2 for 12 minutes to obtain material 3;
...
Embodiment 3
[0069] A composition containing amlodipine besylate, characterized in that: said composition comprises the following components in parts by weight:
[0070] 5 parts of amlodipine besylate; 95 parts of filler; 0.6 parts of disintegrant; 1.3 parts of lubricant.
[0071] The composition is prepared as a tablet.
[0072] The filler is calcium hydrogen phosphate dihydrate.
[0073] The disintegrating agent is 0.2 part of croscarmellose sodium and 0.4 part of crospovidone.
[0074] Described lubricant is magnesium stearate.
[0075] The method for preparing the described composition containing amlodipine besylate is characterized in that, the method may further comprise the steps:
[0076] 1) Ingredients: weigh each component according to the prescription quantity;
[0077] 2) Premix 1: add 3 / 4 filler to amlodipine besylate, mix for 5 minutes, and obtain material 1;
[0078] 3) Sieving: pass material 1 through a 16-mesh sieve to obtain material 2;
[0079] 4) Premix 2: Mix mat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com