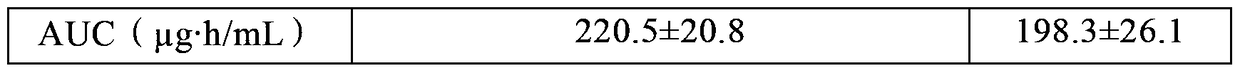A kind of roxithromycin sustained and controlled release preparation
A technology of roxithromycin and controlled-release preparations, which is applied in the field of medicine, can solve problems such as the core material sticking to the surface of microcapsule particles, high one-time investment in equipment, and affecting product quality, so as to achieve good floating performance and improve biological Utilization and the effect of prolonging the residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
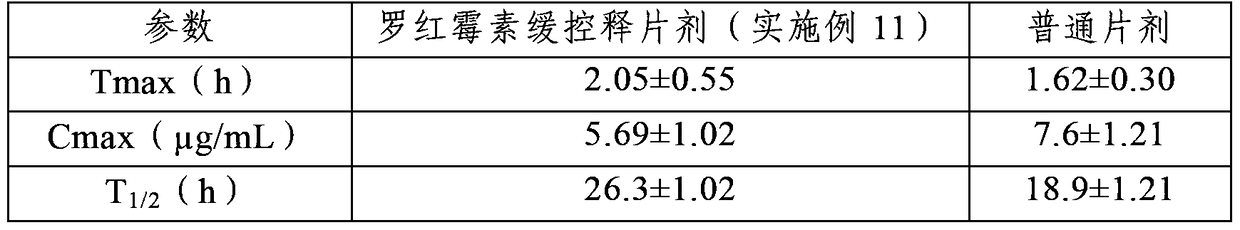
Image
Examples
Embodiment 1
[0029] The roxithromycin sustained-release preparation of the present embodiment is mainly made of roxithromycin and mesoporous silicon dioxide, and the mass ratio of said roxithromycin and mesoporous silicon dioxide is 1:1, and said The mesoporous silica was MCM-48 mesoporous silica.
[0030] The preparation method of the roxithromycin sustained-release preparation of the present embodiment may further comprise the steps:
[0031] Step 1, wet grinding roxithromycin and mesoporous silica together for 3 hours, the grinding medium is water, to obtain drug-loaded silica suspension;
[0032] Step 2, wrapping the drug-loaded silicon dioxide suspension described in step 1 on the microcrystalline cellulose ball core to make micropills by using the liquid phase layering method, and then packing the micropills into capsules to make capsules, Each capsule contains roxithromycin 150mg.
Embodiment 2
[0034] The roxithromycin sustained-release preparation of the present embodiment is mainly made of roxithromycin and mesoporous silicon dioxide, and the mass ratio of said roxithromycin and mesoporous silicon dioxide is 6:1, and said The mesoporous silica was MCM-48 mesoporous silica.
[0035] The preparation method of the roxithromycin sustained-release preparation of the present embodiment may further comprise the steps:
[0036] Step 1, wet-grinding roxithromycin and mesoporous silica for 24 hours, and the grinding medium is water, to obtain a drug-loaded silica suspension;
[0037] Step 2, adding microcrystalline cellulose and lactose to the drug-loaded silicon dioxide suspension described in step 1, mixing uniformly, extruding and spheronizing to make pellets, and then packing the pellets into capsules to make capsules, Each capsule contains roxithromycin 150mg; the quality of microcrystalline cellulose is 30% of the quality of roxithromycin, and the quality of lactose i...
Embodiment 3
[0039] The roxithromycin sustained-release preparation of the present embodiment is mainly made of roxithromycin and mesoporous silicon dioxide, and the mass ratio of said roxithromycin and mesoporous silicon dioxide is 6:1, and said The mesoporous silica was MCM-48 mesoporous silica.
[0040] The preparation method of the roxithromycin sustained-release preparation of the present embodiment may further comprise the steps:
[0041] Step 1, wet grinding roxithromycin and mesoporous silica together for 20 hours, the grinding medium is water, to obtain drug-loaded silica suspension;
[0042] Step 2, adding microcrystalline cellulose and lactose to the drug-loaded silicon dioxide suspension described in step 1, mixing uniformly, extruding and spheronizing to make pellets, and then packing the pellets into capsules to make capsules, Each capsule contains roxithromycin 150mg; the quality of microcrystalline cellulose is 30% of the quality of roxithromycin, and the quality of lactos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com