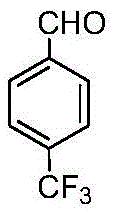
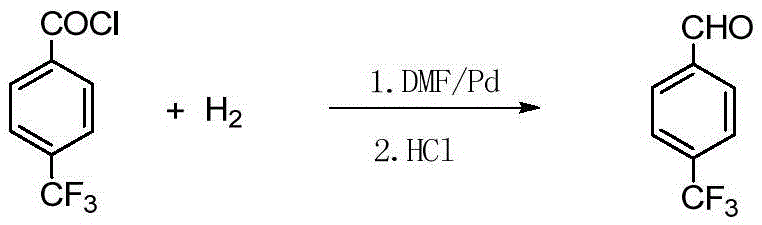Method for preparing p-benzaldehyde
A technology of trifluoromethylbenzaldehyde and trifluoromethylbenzene is applied in the field of preparation of p-trifluoromethylbenzaldehyde, can solve the problems of harsh reaction conditions, unfriendly environment, and high cost of raw materials, and achieves easy post-processing, The effect of cheap raw materials, process flow and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 intermediate 1-nitromethyl-4-trifluoromethylbenzene
[0029] Put 18.0g (0.1mol) of p-chlorobenzotrifluoride and 20.2g (0.2mol) of triethylamine into a 250ml four-neck flask, stir and mix, cool down to -5°C, drop in 30.5g (0.5mol) of nitromethane, drop Bi heat preservation reaction 3h. Add 109.5g (0.3mol) of 10% hydrochloric acid solution after the reaction, extract and separate layers with 100mL toluene, filter, distill to obtain 20.7g1-nitromethyl-4-trifluoromethylbenzene, content 96.9% (liquid chromatography, external standard method).
Embodiment 2
[0030] The preparation of embodiment 2 intermediate 1-nitromethyl-4-trifluoromethylbenzene
[0031] Put 18.0g (0.1mol) of p-chlorobenzotrifluoride and 15.2g (0.15mol) of triethylamine into a 250ml four-neck flask, stir and mix, cool down to -5°C, drop in 12.2g (0.2mol) of nitromethane, drop Bi heat preservation reaction 2h. Add 109.5g (0.3mol) of 10% hydrochloric acid solution after completion of the reaction, extract the layers with 100mL toluene, filter, and distill to obtain 20.6g of 1-nitromethyl-4-trifluoromethylbenzene, with a content of 95.5% (liquid chromatography, external standard method).
Embodiment 3
[0032] The preparation of embodiment 3 intermediate 1-nitromethyl-4-trifluoromethylbenzene
[0033] Put 18.0g (0.1mol) of p-chlorobenzotrifluoride and 10.1g (0.1mol) of triethylamine into a 250ml four-necked flask, stir and mix, cool down to -5°C, drop in 12.2g (0.2mol) of nitromethane, drop Bi heat preservation reaction 2h. Add 109.5g (0.3mol) of 10% hydrochloric acid solution after the reaction, extract the layers with 100mL toluene, filter, and distill to obtain 20.6g of 1-nitromethyl-4-trifluoromethylbenzene, with a content of 95.4% (liquid chromatography, external standard method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com