Method for detecting enantiomer content in levocarnitine
An enantiomer and content technology, applied in the field of drug detection, can solve the problem of high cost of derivatization reagents, and achieve the effects of low cost, high repeatability and accurate detection results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Detection method:
[0046] 1. Chromatographic conditions:
[0047] Chromatographic column: CHIRAL IE chiral column, 250×4.6mm, particle size 5μm; detection wavelength 232nm; mobile phase: n-hexane-absolute ethanol-trifluoroacetic acid (volume ratio 70:30:0.3); column temperature: 30℃ ; Flow rate: 1.0ml / min; Running time: 25min; Injection volume: 10μl;
[0048] Blank solution: Derivative solution without levocarnitine.
[0049] 2. Solution configuration
[0050] 2.1 Configuration of dex-carnitine derivative stock solution:
[0051] Precisely weigh 10 mg of dex-carnitine derivative, put it in a 10ml volumetric flask, dissolve it with absolute ethanol to the mark, shake well, accurately measure 1ml, put it in a 20ml measuring bottle, add absolute ethanol to dilute to the mark, shake well, That is, the concentration is 0.05mg / ml;
[0052] 2.2 Configuration of system adaptability solution:
[0053] Accurately weigh 20mg of levocarnitine derivatives, put it in a 20ml vo...
Embodiment 2
[0061] Using benzoyl chloride as a derivatization reagent to detect the content of enantiomers in levocarnitine:
[0062] 1. Derivatization reaction of L-carnitine
[0063]Weigh 161mg of levocarnitine, put it in a reaction bottle, add 1.6ml of acetic acid and 168mg (1.2 equivalents) of benzoyl chloride, stir at 60-65°C for 0.5 hours, evaporate to dryness under reduced pressure, and obtain levocarnitine benzoyl chloride derivative things.
[0064] 2. Derivatization reaction of dex-carnitine
[0065] Weigh 161 mg of dex-carnitine, put it in a reaction bottle, add 1.6 ml of acetic acid and 168 mg (1.2 equivalents) of benzoyl chloride, stir at 60-65 ° C for 0.5 hours, evaporate to dryness under reduced pressure, and obtain dex-carnitine benzoyl chloride thing.
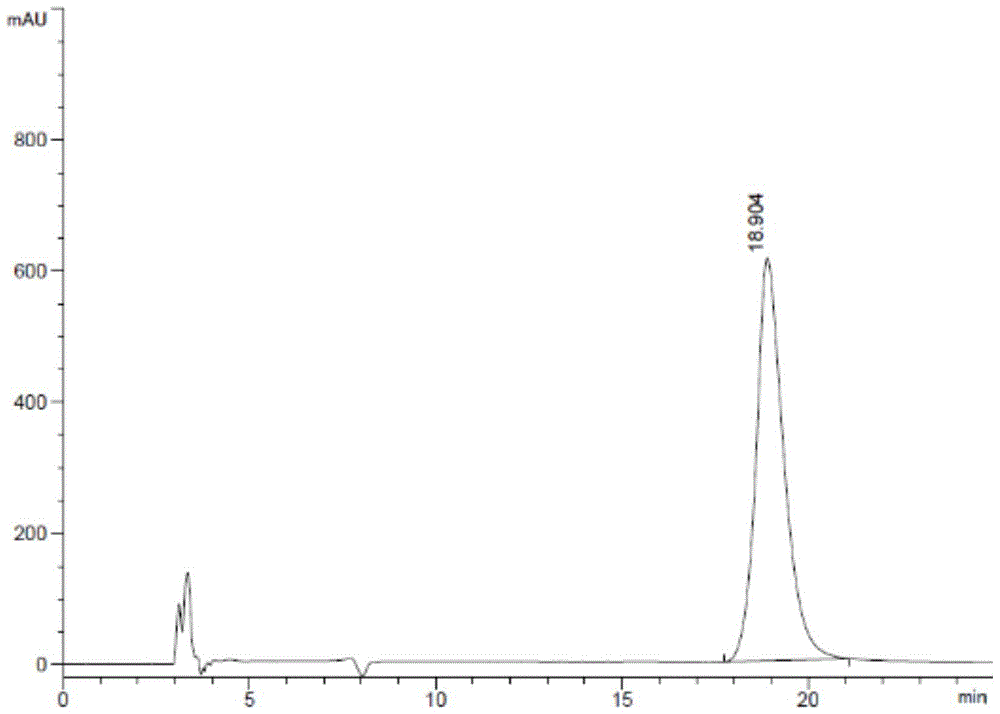
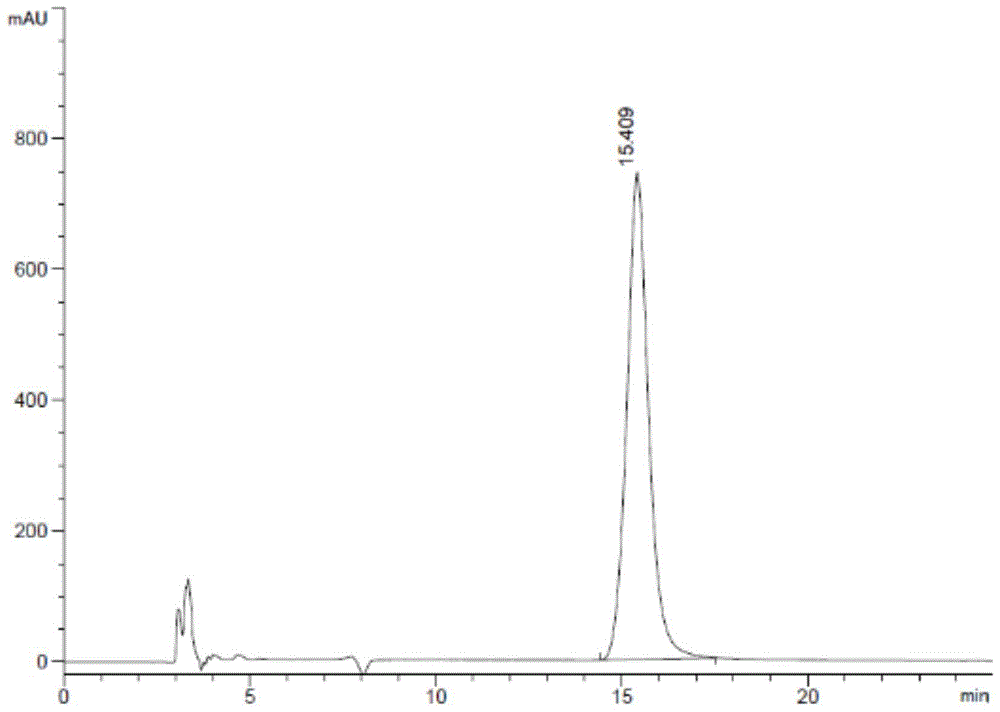
[0066] 3. Detect according to the detection method in Example 1, and the system suitability test results are shown in Table 1:
[0067] Table 1 System suitability test results
[0068]
[0069] See atlas Figures ...
Embodiment 3
[0076] Using p-ethylbenzoyl chloride as a derivatization reagent to detect the content of enantiomers in levocarnitine:
[0077] 1. Derivatization reaction of L-carnitine
[0078] Weigh 200mg of levocarnitine, put it in a reaction bottle, add 1.0ml of trifluoroacetic acid and 312mg (1.5 equivalents) of p-ethylbenzoyl chloride, stir at 65-70°C for 0.5 hours, and evaporate to dryness under reduced pressure to obtain levocarnitine Ting p-ethylbenzoyl chloride derivatives.
[0079] 2. Derivatization reaction of dex-carnitine
[0080] Weigh 200mg of dexcarnitine, put it in a reaction bottle, add 1.0ml of trifluoroacetic acid and 312mg (1.5 equivalent) of p-ethylbenzoyl chloride, stir at 65-70°C for 0.5 hours, evaporate to dryness under reduced pressure, and obtain dexcarnitine Ting p-ethylbenzoyl chloride derivatives.
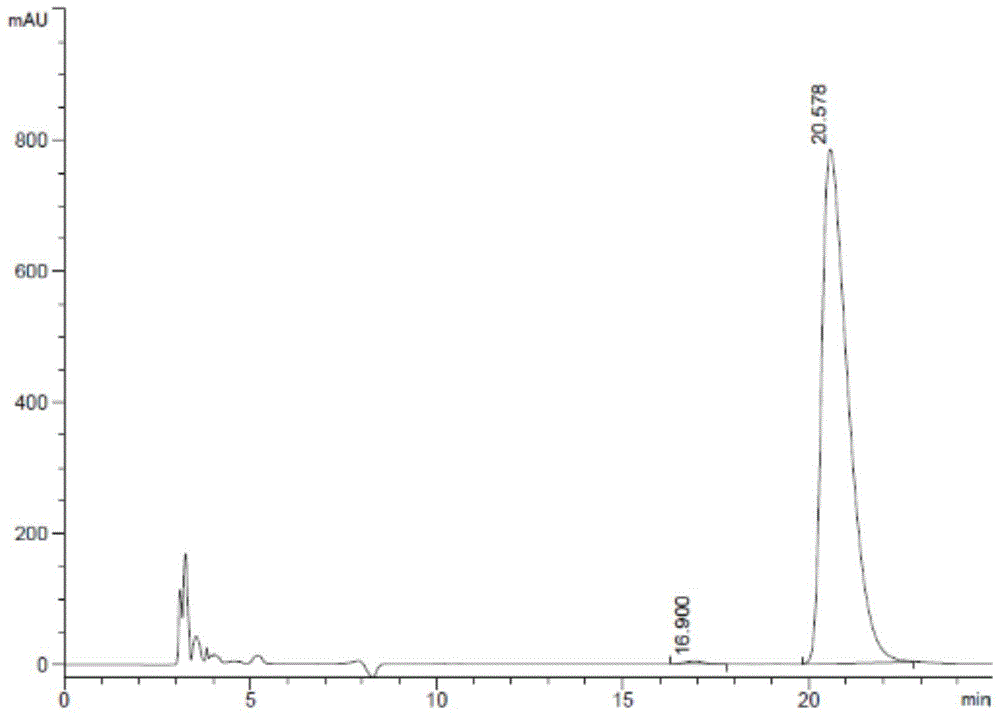
[0081] 3. Detect according to the detection method in Example 1, the enantiomer content in the levocarnitine sample is 0.025wt%, the results are shown in Table 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com