Anti-microbial composition
A technology of antibacterial ingredients and bacteria, applied in the direction of antibacterial drugs, organic active ingredients, ester active ingredients, etc., can solve problems such as treatment problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
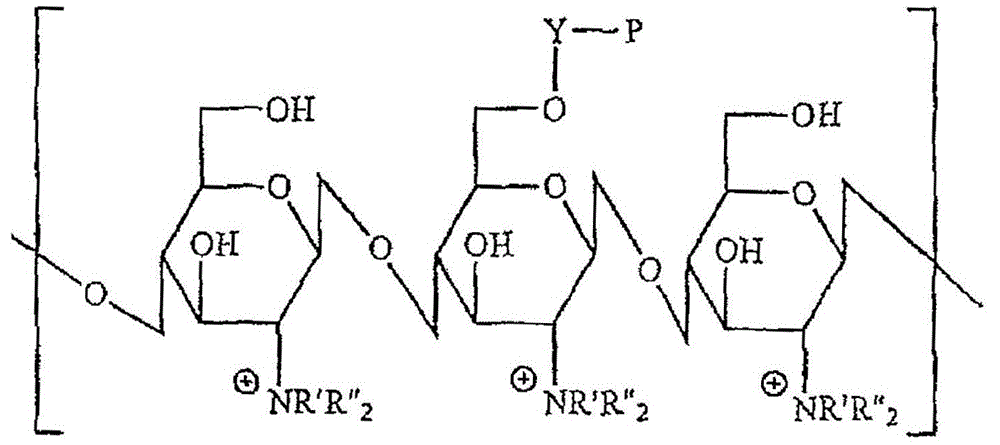
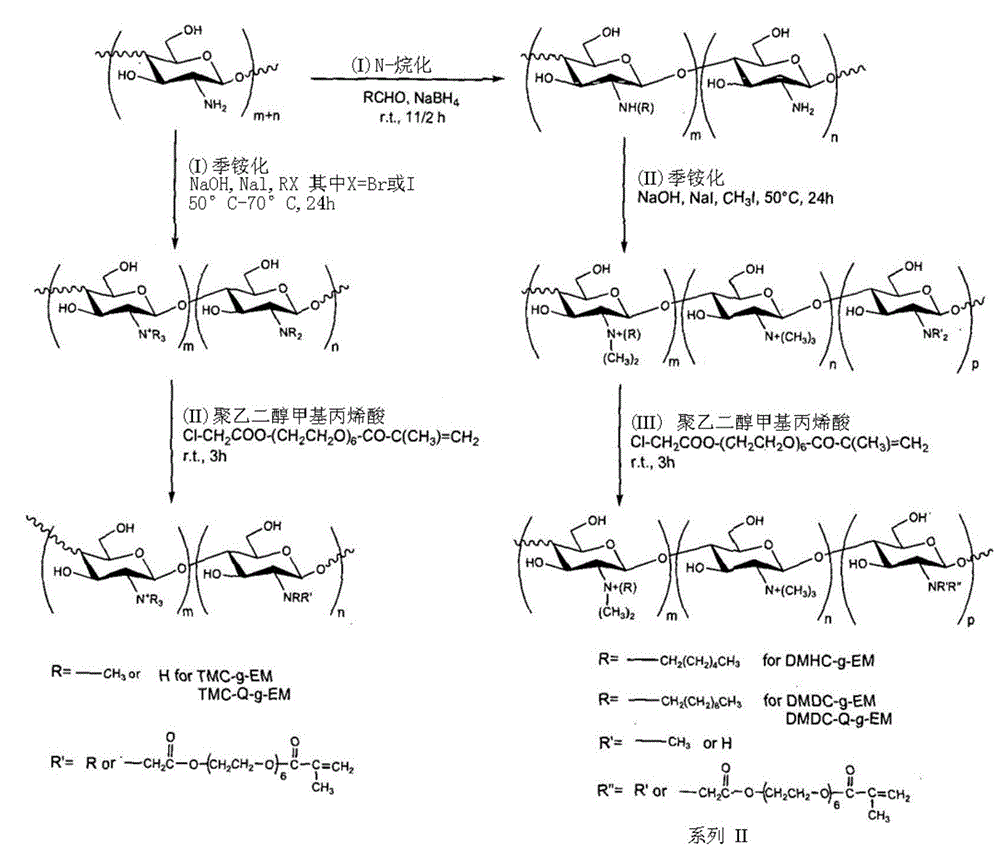
[0048] The typical example of the synthetic method of quaternized chitosan derivative among the present invention comprises the following content:
[0049] 1). The preparation of chlorinated functional polyethylene glycol acrylate derivatives can be realized by the following method, the average molecular weight is 375 polyethylene glycol monoacrylate (2.36ml, 7.06mmol) dissolved in 100ml of toluene , and chloroacetyl chloride (2.25ml, 28.24mmol) was treated in the same manner. The mixture was then heated at reflux for 24 hours. Afterwards, the solvent and volatiles were removed by evaporation, and the residue could be dissolved with dichloromethane (150ml). The solution can then be stirred over potassium carbonate and filtered. The solvent was removed by evaporation and the product was obtained after washing with hexane and dried.
[0050] 2). The preparation method of trimethylammonium chitosan comprises the steps of adding 1 g of chitosan into 50 ml of N-methyl-2-pyrrolid...
example 1
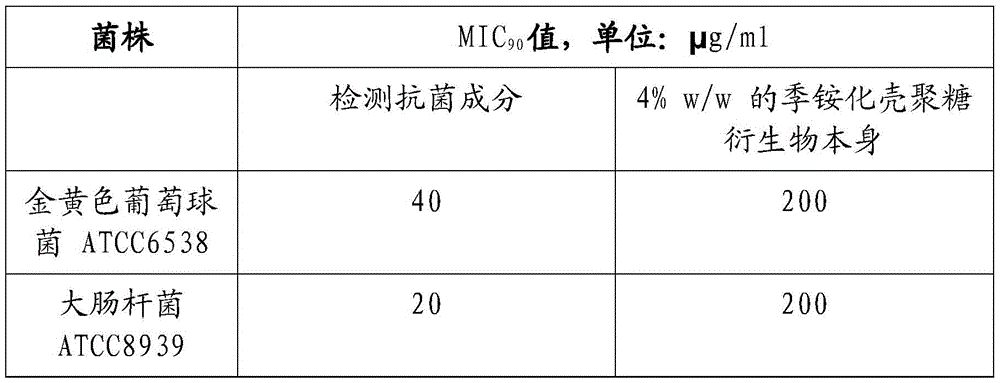
[0138] The MlC of example 1 antibacterial composition 90 determination
[0139] 1% w / v (~10,000ppm) of quaternized chitosan derivatives (DMDC-Q-g-EM) with disodium cocoamphodiacetate (DCC) (concentrations of 5% and 25% in aqueous solution between) to generate experimental antimicrobial components, followed by MIC 90 The method of measurement is verified.
[0140] Briefly, bacterial cells are inoculated in medium (MH broth) and allowed to grow overnight. Spread the bacterial suspension on an agar plate to form dispersed colonies. Subsequent cultures were initiated from single colonies by inoculating purified bacteria in MH broth and grown overnight at 37 °C to reach mid-log phase, followed by dilution to 10 4 to 10 5 CFUml -1 .
[0141] The antimicrobial components were subsequently dissolved in MH broth at the desired concentration. Make a two-fold dilution series of 100 μl of the product solution in MH broth, from No. 1 to No. 10, in a 96-well microplate. Wells ...
example 2
[0153] Example 2 Acute Oral Toxicity in Rats
[0154] The procedure used was derived from the OECD Guidelines for the Testing of Chemicals, Acute Oral Toxicity - Classification Method 423 for Sexual Toxicity.
[0155] For each group of animals tested with an aqueous solution of the tested antimicrobial component, the procedure is as follows:
[0156] 1. Obtain three healthy female nulliparous and non-pregnant Wistar (WI) mice, 6-7 weeks old, and adapt them to the environment of animal toxicity research in the laboratory of National College of Singapore for at least 5 days. All three animals were housed together in an individually ventilated cage (IVC). Room temperature was maintained at 22-26°C, humidity at 40-70%, with a 12-hour light and 12-hour dark cycle. Feed standard laboratory chow for irradiated rodents. Drinking water was provided ad libitum through autoclaved water bottles. Feed (but not water) was left overnight before oral administration of the test substance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com