Enterobacter sakazaii colour development culture medium, detection kit and detection method
A technology of Enterobacter sakazakii and chromogenic medium, which is applied in the field of detection methods and compositions used, can solve the problems of high detection cost, high technical requirements, complicated equipment operation, etc. Sensitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Verification of the specificity of Enterobacter sakazakii chromogenic medium according to the present invention
[0021] 1. Preparation of Enterobacter sakazakii chromogenic medium: Weigh 39.62g of Enterobacter sakazakii chromogenic medium (containing 11g of peptone, 5g of beef extract powder, 5g of sodium chloride, 1.5g of bile salt, 15g of agar, chromogenic medium Substrate X-a-glucopyranoside (Apoloscientific, BIMB1190) 0.1g, Na 2 CO 3 0.02g, 1.0g ferric ammonium citrate, 1.0g sodium thiosulfate), add 1000mL distilled water or deionized water, stir, heat and boil until completely dissolved, autoclave at 121℃ for 15min, cool to 40~50℃, pour plate ,spare;
[0022] 2. Inoculation: Enterobacter sakazakii, Proteus vulgaris, P. mirabilis, Pseudomonas aeruginosa, Escherichia coli, Salmonella typhimurium , Serralia marcescens, Streptococcus faecalis, and Staphylococcus aureus were resuscitated on nutrient agar for 24 hours, respectively streaked and inoculated...
Embodiment 2
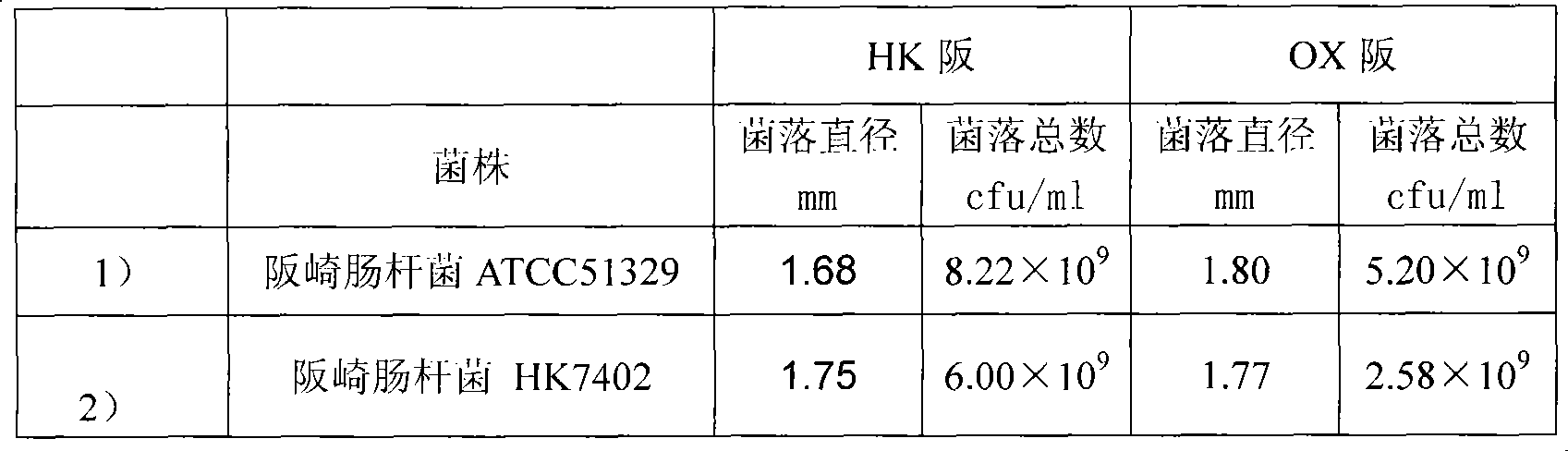
[0024] Example 2: Verification of sensitivity and specificity of Enterobacter sakazakii chromogenic medium according to the present invention
[0025] 1. Preparation of Enterobacter sakazakii chromogenic medium: Weigh 39.62g of Enterobacter sakazakii chromogenic medium (containing 11g of peptone, 5g of beef extract powder, 5g of sodium chloride, 1.5g of bile salt, 15g of agar, chromogenic medium Substrate X-a-glucopyranoside (Apoloscientific, BIMB1190) 0.1g, Na 2 CO 3 0.02g, 1.0g ferric ammonium citrate, 1.0g sodium thiosulfate), add 1000mL distilled water or deionized water, stir, heat and boil until completely dissolved, autoclave at 121℃ for 15min, cool to 40~50℃, pour plate ,spare;
[0026] 2. Inoculation: Enterobacter sakazakii ATCC51329, HK7402, Proteus vulgaris CMCC (B) 49027, Proteus mirabilis CMCC (B) 49005, Pseudomonas aeruginosa ATCC9027, ATCC27853, Escherichia coli ATCC25922, Salmonella typhimurium CMCC (B) 50115, Serratia marcescens HK7121, Enterococcus faecal...
Embodiment 3
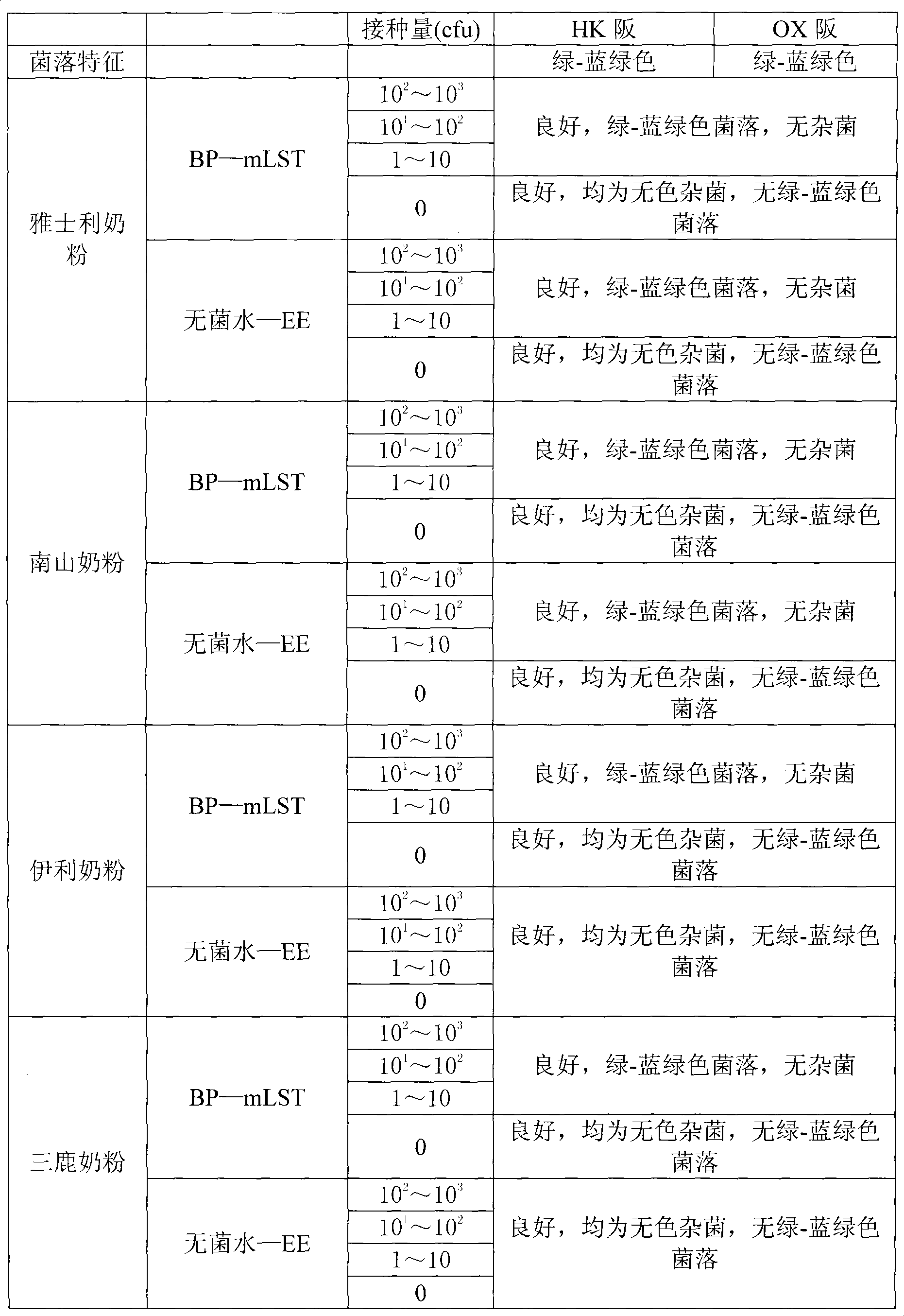
[0038] Example 3: Detection of Enterobacter sakazakii in artificially contaminated samples
[0039] 1. Preparation of chromogenic plate
[0040] Weigh 39.62g of chromogenic medium dry powder (containing peptone 11g, beef extract powder 5g, sodium chloride 5g, bile salt 1.5g, agar 15g, chromogenic substrate X-a-glucopyranoside (Apoloscientific, BIMB 1190) 0.1g, Na 2 CO 3 0.02g, 1.0g ferric ammonium citrate, 1.0g sodium thiosulfate), add 1000mL distilled water or deionized water, stir, heat and boil until completely dissolved, autoclave at 121℃ for 15min, cool to 40~50℃, pour plate ,spare;
[0041] 2. Pre-enrichment bacteria
[0042] Weigh 8 parts of each of the 4 milk powder samples, each 100g, and add 4 samples to the triangular flask containing 900mL BP enrichment solution by aseptic operation, 3 of which are added with three concentrations of Enterobacter sakazakii; the other 4 The samples were added to Erlenmeyer flasks containing 900mL of sterile water, three of which...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com