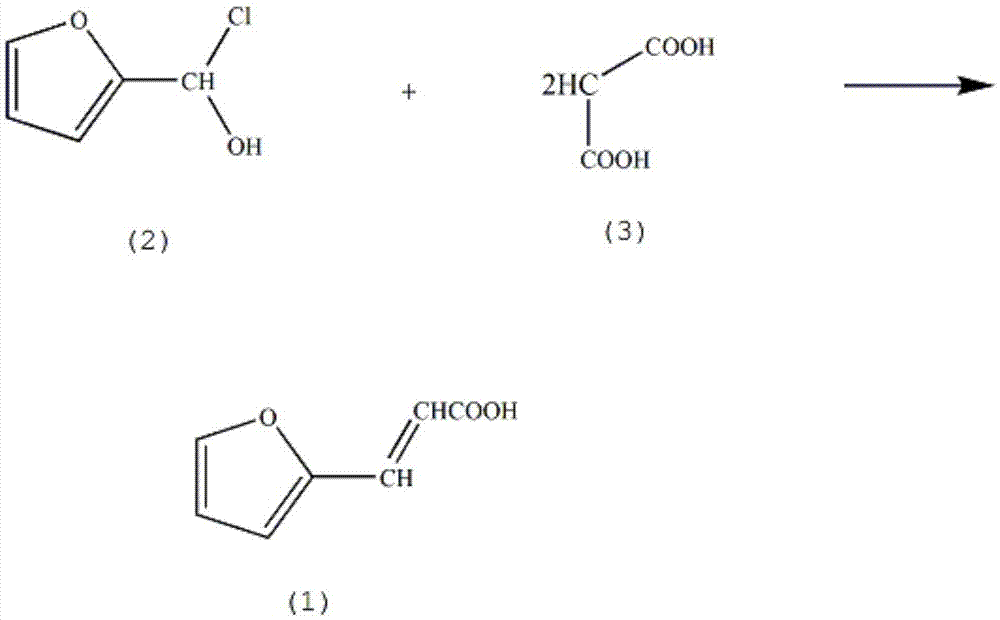
Synthesis method of furapromide drug intermediate 2-furfuracrylic acid
A technology of furan acrylic acid and furan propylamine, applied in directions such as organic chemistry, can solve problems such as poor curative effect, and achieve the effects of reducing intermediate links, improving reaction yield, reducing reaction temperature and reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0011] In the reaction vessel that stirrer, reflux condenser are installed, adding mass fraction is 70% 2-furan chloromethanol solution (2) 3.1mol, mass fraction is 80% malonic acid solution (3) 3.6mol, mass fraction is 300ml of 60% nitroethane, control the stirring speed at 130rpm, increase the solution temperature to 70°C, reflux for 5h, lower the solution temperature to 8°C, add 310ml mass fraction of 30% sodium bromide solution, 200ml mass fraction of 40% sodium bromide solution Potassium bisulfate solution, reacted for 90min, stood still for 3h, precipitated solid, filtered, washed with ammonium bromide solution, the mass fraction was 70% toluene, 85% ethylenediamine was washed, activated alumina was dehydrated, and the mass fraction was Recrystallized from 91% acrylonitrile to obtain 355.07 g of crystal 2-furan acrylic acid, with a yield of 83%.
example 2
[0013] In the reaction vessel that agitator and reflux condenser are installed, adding mass fraction is 73% 2-furan chloromethanol solution (2) 3.1mol, mass fraction is 82% malonic acid solution (3) 3.8mol, mass fraction is 300ml of 63% nitroethane, control the stirring speed at 140rpm, increase the solution temperature to 72°C, reflux for 6h, lower the solution temperature to 10°C, add 310ml of 32% sodium bromide solution, and 200ml of 42% sodium bromide solution. Potassium bisulfate solution, reacted for 110min, stood still for 5h, precipitated solid, filtered, washed with potassium iodide solution, with mass fraction of 72% toluene, mass fraction of 87% ethylenediamine, anhydrous sodium carbonate dehydration, at mass fraction of 97% % acrylic acid was recrystallized to obtain 367.91 g of crystalline 2-furan acrylic acid, with a yield of 86%.
example 3
[0015] In the reaction vessel that agitator and reflux condenser are installed, adding mass fraction is 76% 2-furan chloromethanol solution (2) 3.1mol, mass fraction is 87% malonic acid solution (3) 3.9mol, mass fraction is 300ml of 66% nitroethane, control the stirring speed at 170rpm, increase the solution temperature to 75°C, reflux for 8h, lower the solution temperature to 13°C, add 310ml of 35% sodium bromide solution, and 200ml of 45% sodium bromide solution. Potassium bisulfate solution, reacted for 120min, stood still for 6h, precipitated solid, filtered, washed with ammonium bromide solution, the mass fraction was 75% toluene washing, the mass fraction was 90% ethylenediamine washing, activated alumina dehydration, the mass fraction was Recrystallized from 98% acrylonitrile to obtain 389.30 g of crystalline 2-furanacrylic acid, with a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com