A kind of clean preparation method of layered composite hydroxide
A hydroxide, layered composite technology, applied in the preparation of organic compounds, cyanide reaction preparation, oxygen/ozone/oxide/hydroxide, etc., can solve the problem of high energy consumption, long heating time and high reaction temperature problem, to achieve the effect of simple preparation process, low price and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Step A: Weigh 8.4g of calcium oxide and add it to 100g of deionized water, grind it in a ball mill for 2 minutes to prepare a calcium oxide slurry, and then add it to reactor A; weigh 16.05g of NH 4 Cl was added to 100ml deionized water to prepare a solution and added to reactor A, and stirred evenly.
[0026] Step B: Weigh 8.12g MgCl 2 . 6H 2 O and 4.826g AlCl 3 . 6H 2 O was added to 100ml of deionized water to prepare a salt solution and added to reactor B. Weigh 6.42gNH 4 Cl was added to 100ml deionized water to prepare a solution and added to reactor B, and stirred evenly.
[0027] C: Connect reactor A and reactor B with a condensing device, heat reactor A to 100°C and reactor B to 50°C under stirring. After 3 hours, the reactor A stopped heating and closed the condensing device, heated the reactor B to 100°C, continued to stir and react for 4 hours, cooled to room temperature after the reaction, and the precipitate was washed by centrifugation until the pH ...
Embodiment 2
[0029] Step A: Weigh 8.4g of calcium oxide and add it to 80g of deionized water, grind it in a ball mill for 1 minute, prepare calcium oxide slurry, and then add it to reactor A; weigh 24g of NH 4 NO 3 Add it into 120ml deionized water to prepare a solution and add it to reactor A, and stir evenly.
[0030] Step B: Weigh 12.18g MgCl 2 . 6H 2 O and 4.826g AlCl 3 . 6H 2 O was added to 100ml of deionized water to prepare a salt solution and added to reactor B. Weigh 2.64g (NH 4 ) 2 SO 4 Add it to 50ml deionized water to make a solution and add it to reactor B, and stir evenly.
[0031] C: Connect reactor A and reactor B with a condensing device, heat reactor A to 90°C and reactor B to 60°C under stirring. After 6 hours, the reactor A stopped heating and closed the condensing device, heated the reactor B to 90°C, continued to stir and react for 3 hours, cooled to room temperature after the reaction, the precipitate was washed by centrifugation until the pH value was les...
Embodiment 3
[0033] Step A: Same as Example 2.
[0034] Step B: with embodiment 2, difference is to take by weighing 1.92g (NH 4 ) 2 CO 3 Instead of the used (NH 4 ) 2 SO 4 .
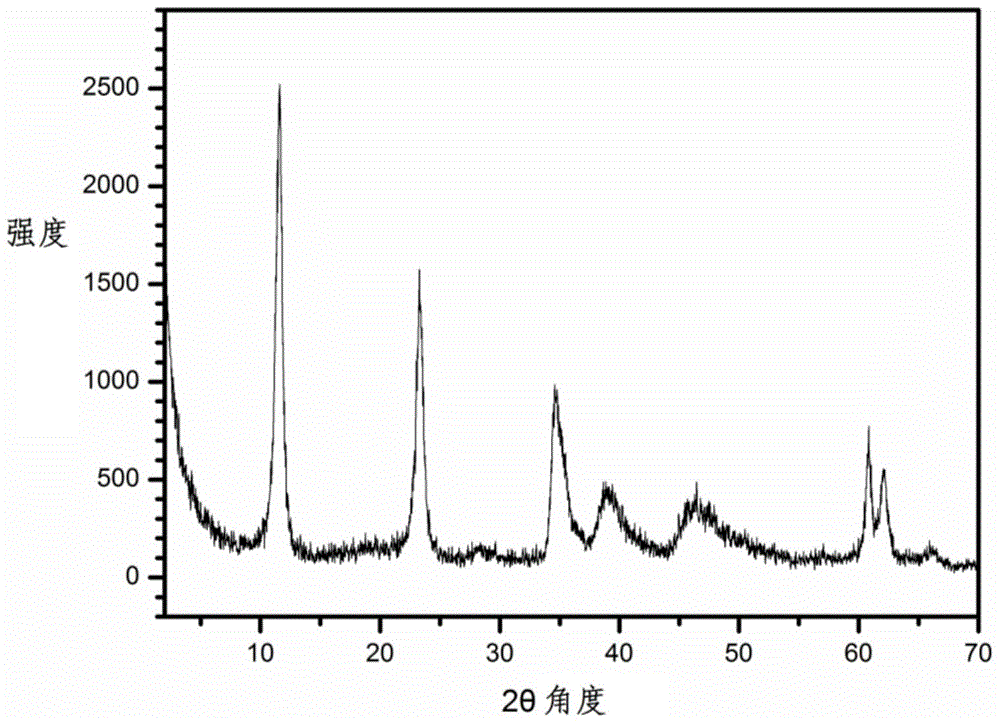
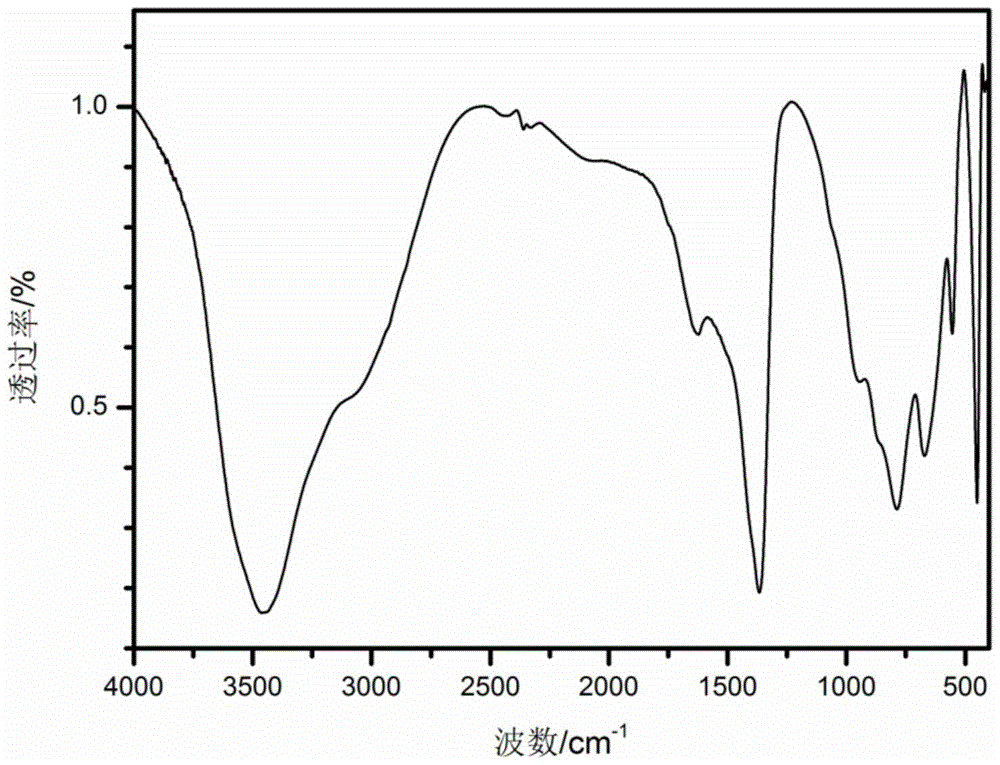
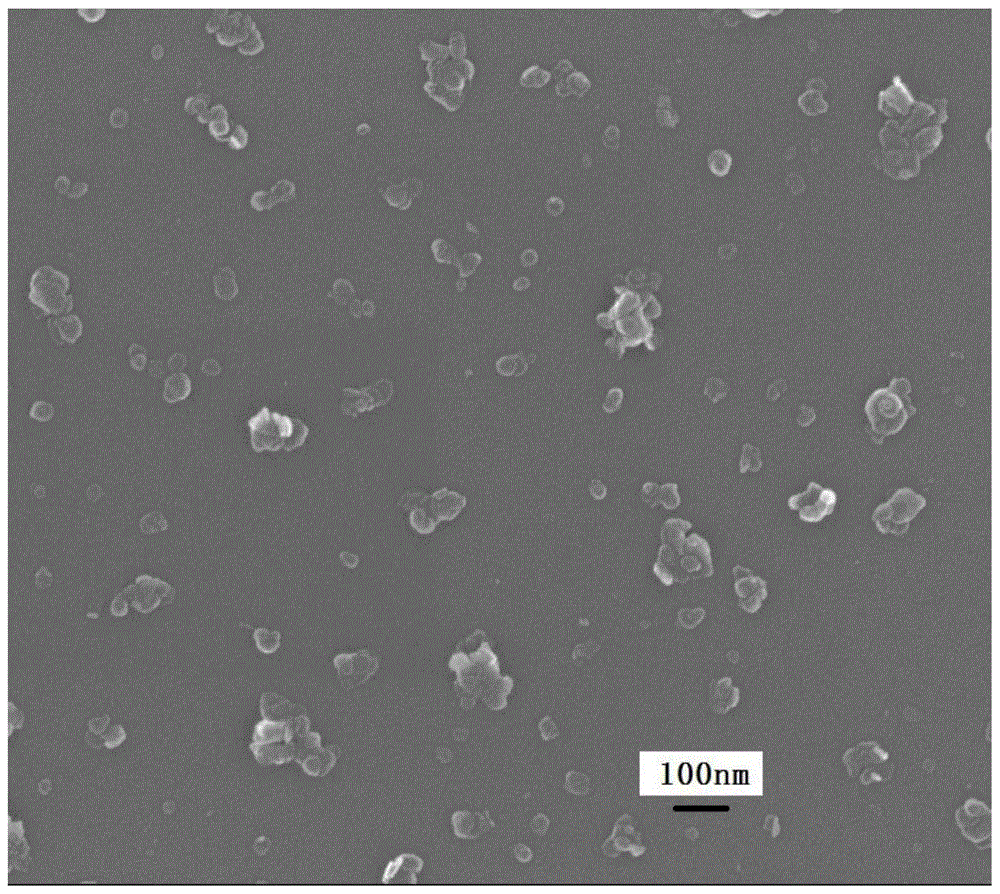
[0035] C: Connect reactor A and reactor B with a condensing device, heat reactor A to 95°C and reactor B to 50°C under stirring. After 5 hours, stop the heating of reactor A and close the condensing device, heat reactor B to 80 ° C, continue to stir for 4 hours, cool to room temperature after the reaction, the precipitate is washed by centrifugation until the pH value is less than 8, and the sample is placed in Dry in an oven at 100°C for 12 hours to obtain the LDH product. Elemental analysis shows that the chemical composition formula of the product is: Mg 0.75 Al 0.25 (OH) 2 (CO 3 ) 0.125 0.8H 2 O.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com