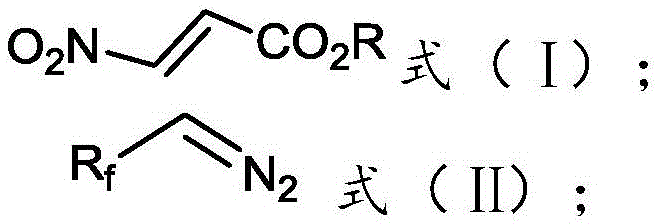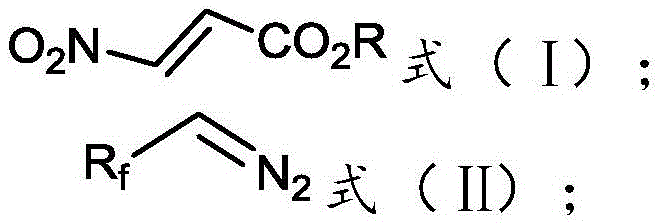Preparation method of 3-fluoroalkyl-1H-pyrazole-4-formic ester
A fluoroalkyl and formate technology, applied in the field of organic compound preparation, can solve the problems of low regioselectivity and yield, expensive and difficult raw materials, limited application scope, etc., and achieves mild reaction conditions and substrate universality. Good, high-purity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The invention provides a preparation method of 3-fluoroalkyl-1H-pyrazole-4-carboxylate, comprising:
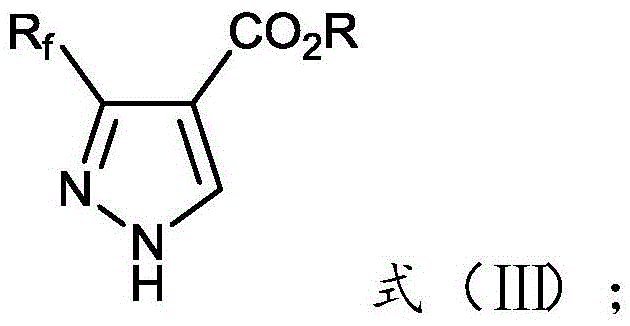
[0019] Mix and react the 3-nitroacrylate shown in formula (I), the fluoroalkyl diazo compound shown in formula (II), catalyst, and basic compound in an organic solvent to obtain the compound shown in formula (III) 3-fluoroalkyl-1H-pyrazole-4-carboxylate;
[0020]
[0021]
[0022] Among them, R f is difluoromethyl, trifluoromethyl or pentafluoroethyl; R is methyl, ethyl, n-propyl, butyl, phenyl or benzyl.
[0023] The preparation method provided by the invention has simple operation steps, mild reaction conditions, high yield, good substrate universality and high purity of the product.
[0024] Above-mentioned reaction equation is as follows:
[0025]
[0026] Among them, R f Preference is given to difluoromethyl, trifluoromethyl or pentafluoroethyl.
[0027] R is preferably methyl, ethyl, n-propyl, butyl, phenyl or benzyl.
[0028] The catalyst is prefer...
Embodiment 13- 3
[0038] Preparation of Example 13-trifluoromethyl-1H-pyrazole-4-carboxylic acid ethyl ester
[0039]
[0040] Weigh Ag into a dry 100mL Schlenk reaction flask 2 O (0.17g, 0.75mmol), Na 3 PO 4 (0.12g, 0.75mmol), ethyl 3-nitroacrylate (0.73g, 0.5mmol), replace argon 3 times, add CF to the system 3 CHN 2 THF solution (0.2M, 25mL), stirred at room temperature for 3h. The reaction was complete as monitored by TLC, the metal and inorganic salts in the system were removed by filtration, the filter residue was washed with ethyl acetate, the organic phases were combined, the solvent was evaporated under reduced pressure, the residue was dissolved in dichloromethane, washed with saturated ammonium chloride solution, anhydrous sodium sulfate Drying, column chromatography (eluent: petroleum ether / ethyl acetate=10 / 1~5 / 1) can obtain target product 3-trifluoromethyl-1H-pyrazole-4-carboxylic acid ethyl ester 0.65g, The yield was 72%, and the high performance liquid chromatography (HPLC...
Embodiment 23- 3
[0045] Preparation of Example 23-trifluoromethyl-1H-pyrazole-4-carboxylic acid methyl ester
[0046]
[0047] Using the same method as in Example 1, using silver carbonate as a catalyst, sodium carbonate as a base, and dichloromethane as a solvent, 3-methyl nitroacrylate, silver carbonate, sodium carbonate and 2,2,2-trifluorodiazoethyl Alkanes were mixed in a molar ratio of 1 / 3 / 3 / 8, reacted at 40°C for 12h, and synthesized 3-trifluoromethyl-1H-pyrazole-4-carboxylic acid methyl ester, and the aftertreatment was the same as in Example 1, with a yield of 70 %, high performance liquid chromatography (HPLC) purity of 99%.
[0048] The structure of the product was detected by nuclear magnetic resonance, and the results were as follows:
[0049] 1 HNMR (400MHz, CDCl 3 )δ13.32(s,1H), 8.17(s,1H), 4.18(s,3H).
[0050] 19 FNMR (376MHz, CDCl 3)δ-61.79(s).
[0051] 13 CNMR (100MHz, CDCl 3 ) δ 162.2, 141.9 (q, J=37.9Hz), 138.7, 122.1 (q, J=268.0Hz), 112.5, 59.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com