Two NK007 optical pure isomers and synthesis and application thereof
A technology of optical isomers and isomers, applied in applications, botany equipment and methods, chemicals for biological control, etc., can solve problems such as increased transportation and storage costs, strong odor, and environmental pollution, and achieve Good effect on regulating immune activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of Compounds 6, 9a, 11a and Compound NK007-1, Compound NK007-2
[0038] Compound 6 (such as image 3 shown)
[0039] N 2 Under protection, 150 mL of freshly distilled anhydrous THF was added to a 250 mL reaction flask, and 1.14 g (0.03 mol) of NaBH was added in batches at room temperature. 4 6.84 g (0.02 mol) of raw material 5 was added in batches under electromagnetic stirring, stirred for 10 minutes, 6.99 g (0.03 mol) of zirconium tetrachloride was slowly added, stirred at room temperature for 10 hours, and the reaction of raw materials was detected by TLC. Add 10mL of water and 10mL of dilute hydrochloric acid dropwise under cooling in an ice-water bath to fully decompose excess NaBH 4 , after precipitation with 250mLCH 2 Cl 2 Dilute with 50mL of water, stir and separate, the organic phase is washed with water, washed with saturated brine, anhydrous MgSO 4 Drying and desolvation gave 6.46 g of a white solid product with a yield of 95%. Mel...
Embodiment 2
[0049] Embodiment 2: the anti-tobacco mosaic virus activity (table 2) of compound NK007-1 and NK007-2
[0050] 1. Virus purification and concentration determination:
[0051] Virus purification and concentration determination were carried out in accordance with the SOP specification for tobacco mosaic virus compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the crude virus extract was centrifuged twice with polyethylene glycol, the concentration was measured and refrigerated at 4°C for later use.
[0052] 2. Compound solution preparation:
[0053] After weighing, the original drug was dissolved in DMF to prepare 1×10 5 μg / mL mother solution, and then diluted with 1‰ Tween 80 aqueous solution to the required concentration; Ningnanmycin preparation was directly diluted with water.
[0054] 3. In vitro effect:
[0055] Rub inoculation of leaves of Shanxi tobacco at the right age, rinse with running water, the virus concentration is 10 μ...
Embodiment 3
[0068] Example 3: Modulating immune activity of compounds NK007-1 and NK007-2
[0069] testing method
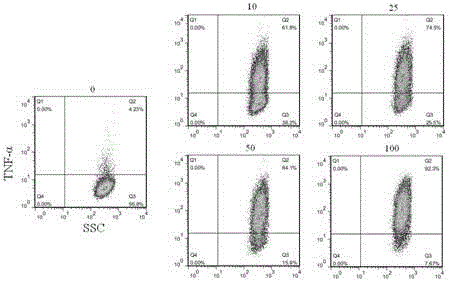
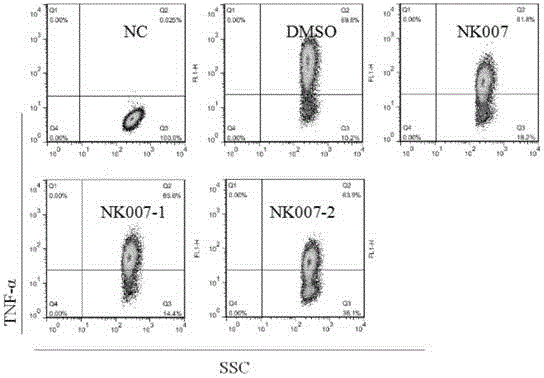
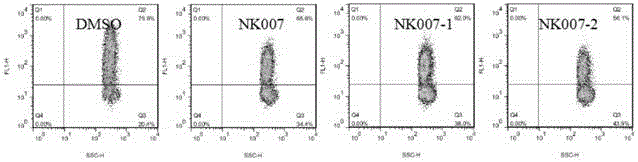
[0070]Materials: 2 sets of surgical scissors, 2 sets of ophthalmic forceps, glass slides, 96-well plate (Corning Company), pipette, 40 μm cell strainer, 15 mL centrifuge tube (BD Company), EP tube, counting plate, microscope, centrifuge, discharge gun, Babl / c mice (Victorian Liver). Reagents: PBS (Beijing Suo Lai Bao Company), RPMI-1640 medium and DMEM medium (Gibco Company), serum (Gibco Company), erythrocyte lysate (Beijing Suo Lai Bao Company), LPS (Sigma Company); Trypan blue. Double antibody (Beiyuntian Company) (1) Effect of optical isomers on the production of TNF-α in splenocytes stimulated by LPS
[0071] Steps:
[0072] 1) Configuration of various solutions: NK007 optical isomer mother solution is 1mM;
[0073] LPS: 1mg / mL;
[0074] Complete medium: 1640+10% serum+1% double antibody.
[0075] 2) Preparation of splenocyte suspension:
[0076] 1. Rat killing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com