Gold@zinc oxide core-shell heterogeneous nanoparticles having strong sunlight absorption property, and preparation method thereof
A gold zinc oxide and nanoparticle technology, which is applied in the field of gold zinc oxide (AuZnO) core-shell heterogeneous nanoparticles and its preparation, can solve the problems of low absorption efficiency, insufficient preparation technology of core-shell heterogeneous composite nanoparticles, electron-space Holes are easy to recombine and other problems, achieving the effect of uniform particle size, good photocatalytic degradation characteristics, and simple and easy-to-operate process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of 80nm gold zinc oxide (AuZnO) core-shell heterogeneous composite nanoparticles
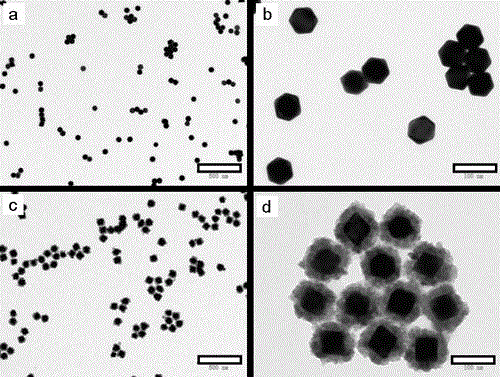
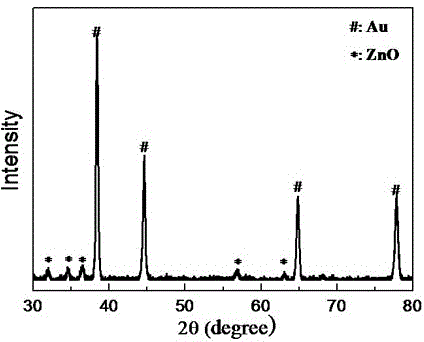
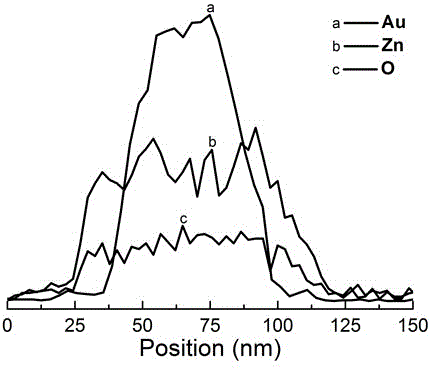
[0034] First add diethylene glycol diacrylate (PDDA, Mw=100000-200000, 20wt%) aqueous solution to the ethylene glycol solution, stir well to obtain a mixed solution; then add chloroauric acid to the mixed solution (HAuCl 4 ) aqueous solution, diethylene glycol diacrylate (PDDA), chloroauric acid (HAuCl 4 ) concentrations are respectively 0.025 mol / L and 0.0005 mol / L; subsequently, the prepared solution was placed at 220°C for 30 minutes to obtain a colloidal solution of purple-red gold (Au) octahedral nanoparticles, the size of which is 60 nm, such as figure 1 a. figure 1 b. After naturally cooling to room temperature, 18 MΩ deionized water (H 2 O), zinc nitrate (Zn(NO 3 ) 2 ), sodium hydroxide (NaOH) and sodium borohydride (NaBH 4 ) aqueous solution to obtain a reaction precursor solution for preparing gold zinc oxide (AuZnO) core-shell heterogeneous composite nanopa...
Embodiment 2
[0036] Preparation of 90nm gold zinc oxide (AuZnO) core-shell heterogeneous composite nanoparticles
[0037] Under the condition of magnetic stirring at room temperature, 18 megohm deionized water (H 2 O), zinc nitrate (Zn(NO 3 ) 2 ), sodium hydroxide (NaOH) and sodium borohydride (NaBH 4 ) aqueous solution to obtain a reaction precursor solution for preparing gold zinc oxide (AuZnO) core-shell heterogeneous composite nanoparticles, wherein water (H in the reaction precursor solution 2 The volume percentage of O) is 20%, zinc nitrate (Zn(NO 3 ) 2 ), sodium hydroxide (NaOH), sodium borohydride (NaBH 4 ) concentrations were 0.0008 mol / L, 0.004 mol / L, and 0.0008 mol / L; the reaction precursor solution was reacted at 60 degrees for 20 minutes to obtain a colloidal solution of gold zinc oxide (AuZnO) core-shell heterogeneous composite nanoparticles; After centrifuging with a high-speed centrifuge at a speed of 9000 rpm for 20 minutes, remove the colorless solution in the cen...
Embodiment 3
[0039] Preparation of 100nm gold zinc oxide (AuZnO) core-shell heterogeneous composite nanoparticles
[0040] Under the condition of magnetic stirring at room temperature, 18 megohm deionized water (H 2 O), zinc nitrate (Zn(NO 3 ) 2 ), sodium hydroxide (NaOH) and sodium borohydride (NaBH 4 ) aqueous solution to obtain a reaction precursor solution for preparing gold zinc oxide (AuZnO) core-shell heterogeneous composite nanoparticles, wherein water (H in the reaction precursor solution 2 The volume percentage of O) is 20%, zinc nitrate (Zn(NO 3 ) 2 ), sodium hydroxide (NaOH), sodium borohydride (NaBH 4 ) concentrations were 0.0016 mol / L, 0.008 mol / L, and 0.0016 mol / L; the reaction precursor solution was reacted at 60 degrees for 25 minutes to obtain a colloidal solution of gold zinc oxide (AuZnO) core-shell heterogeneous composite nanoparticles; Use a high-speed centrifuge to centrifuge at a speed of 8000 rpm for 20 minutes, remove the colorless solution in the centrifu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Scale | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com