A method for catalytic conversion of synthesis gas
A catalytic conversion and synthesis gas technology, applied in chemical instruments and methods, carbon monoxide reaction preparation, physical/chemical process catalysts, etc., can solve the problem of the preparation of cyclohexanone and the simultaneous generation of cyclohexanone and hydrocarbons that have not been seen and other problems, to achieve the effect of stable structure, stable performance and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
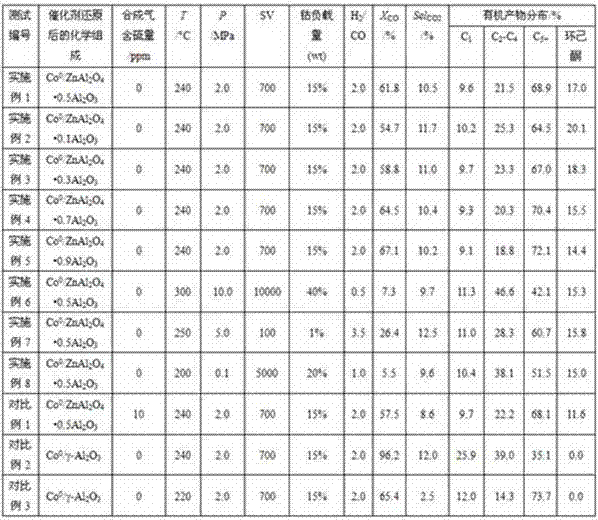
Embodiment 1
[0026] Weigh 26.1880 grams of Co(NO 3 ) 2 ·6H 2 O, 38.1517 g Zn(NO 3 ) 2 ·6H 2 O and 144.3260 g Al(NO 3 ) 3 9H 2 O, dissolved in 500 milliliters of deionized water, was prepared into solution A containing Co, Zn, and Al elements at the same time. Weigh 152.1399 grams of NaHCO 3 , dissolved in 1800 ml of deionized water to prepare solution B. Under vigorous stirring at 40°C, drip solution A and solution B into a beaker to implement co-precipitation reaction, continue stirring and aging at 40°C for 1 hour after the solution is dripped, filter with suction, wash with deionized water, and repeat washing and filtration Until the filtrate does not contain sodium ions, dry the filter cake in an oven at 80°C for 4 hours to semi-dry, add a small amount of scallop powder (extrusion aid), knead, extrude into strips and dry them in the oven at 110°C Bake for 2 hours until dry, then calcinate in a muffle furnace at 350°C for 4 hours, take it out, break it, and pass through a 40-6...
Embodiment 2
[0028] Adopt the method for preparing catalyst by coprecipitation described in embodiment 1, make unreduced ZnAl by adjusting the proportioning consumption of reagent 2 o 4 •xAl 2 o 3 (x=0.1) Carrier loaded cobalt solid phase material, named C2. Expressed in the format "Substance / Carrier", the chemical composition of sample C2 is Co 3 o 4 / ZnAl 2 o 4 •0.1Al 2 o 3 . Pack sample C2 in a fixed-bed single-tube reactor under 10% H 2 Reduction at 600°C for 2h in -90% Ar atmosphere to produce reduced ZnAl 2 o 4 •xAl 2 o 3 (x=0.1) Carrier loaded cobalt solid phase material, named C2R. Expressed in the format of "support / carrier", the chemical composition of the sample C2R is Co 0 / ZnAl 2 o 4 •0.1Al 2 o 3 . Metallic cobalt in sample C2R (i.e. Co 0 phase) with a weight content of 15%. Carrier (ZnAl 2 o 4 •0.1Al 2 o 3 ) is 85% by weight, and the molar ratio of Zn element to Al element in the carrier is 1:2.2. Cool down to room temperature (25°C) after reducing ...
Embodiment 3
[0030] Adopt the method for preparing catalyst by coprecipitation described in embodiment 1, make unreduced ZnAl by adjusting the proportioning consumption of reagent 2 o 4 •xAl 2 o 3 (x=0.3) Carrier loaded cobalt solid phase material, named C3. Expressed in the format "Substance / Carrier", the chemical composition of sample C3 is Co 3 o 4 / ZnAl 2 o 4 •0.3Al 2 o 3 . Pack sample C3 in a fixed-bed single-tube reactor under 50% H 2 -50%N 2 Reduced at 400°C for 50h in the atmosphere to produce reduced ZnAl 2 o 4 •xAl 2 o 3 (x=0.3) Carrier loaded cobalt solid phase material, named C3R. Expressed in the format of "support / carrier", the chemical composition of the sample C3R is Co 0 / ZnAl 2 o 4 •0.3Al 2 o 3 . Metallic cobalt in sample C3R (i.e. Co 0 phase) with a weight content of 15%. Carrier (ZnAl 2 o 4 •0.3Al 2 o 3 ) is 85% by weight, and the molar ratio of Zn element to Al element in the carrier is 1:2.6. Reduce the temperature of sample C3 to 60°C and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com