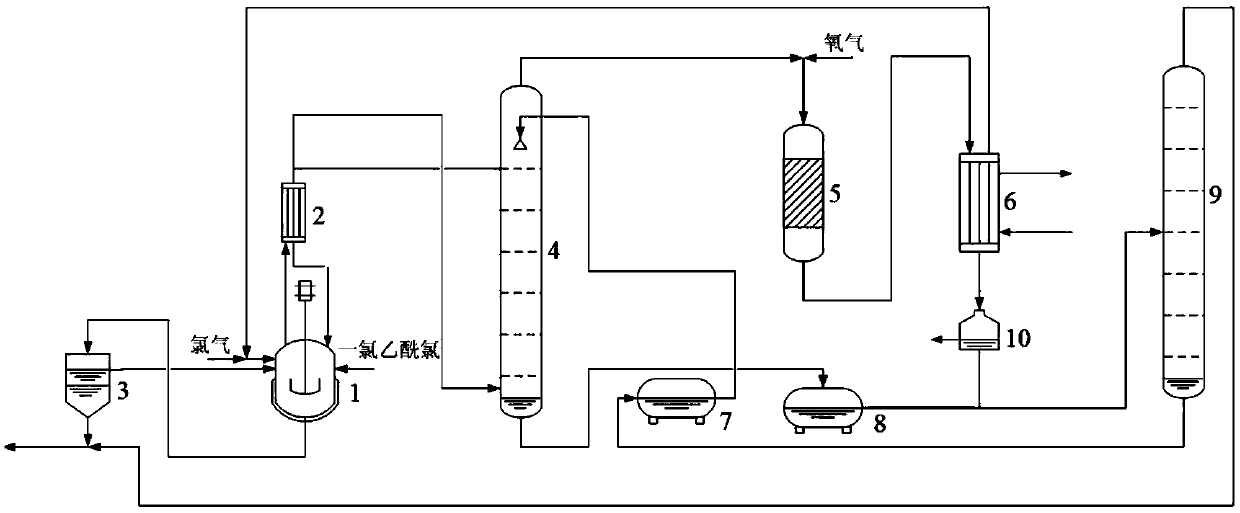
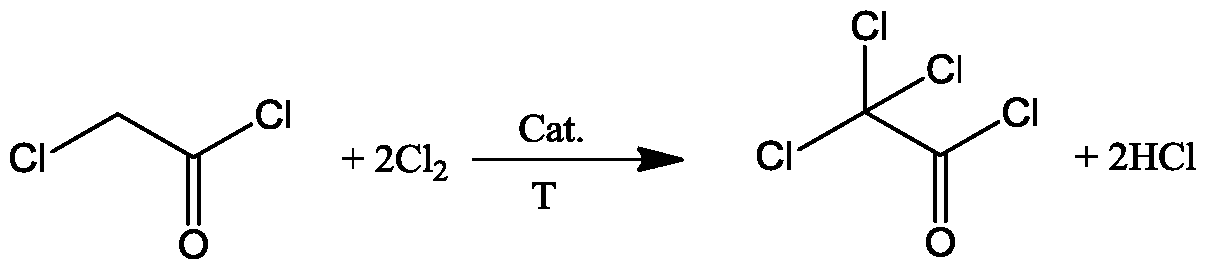
Chlorine resource recycling method and system for chloroacetyl chloride chlorination reaction process
A technology of chlorination reaction and monochloroacetyl, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as waste of raw materials, achieve elimination of oxygen separation process, reduce equipment investment costs, and simplify process routes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] at 10m 3 Add 11 tons of monochloroacetyl chloride into the chlorination reaction kettle, and add 220kg of pyridine hydrochloride at the same time, at 90°C, feed 387kg / h fresh chlorine gas to carry out pre-chlorination reaction. The conversion rate of chloroacetyl chloride reaches about 98%, and the mass fraction of unreacted monochloroacetyl chloride in the reaction solution is about 2.2wt%, and the mass fraction of dichloroacetyl chloride obtained by chlorination is about 0.2wt%. When the mass fraction of chloroacetyl chloride is about 97.6wt%, start to carry out continuous chlorination reaction, with the mass flow rate of 387kg / h, fresh chlorine, the mass flow rate of 576.8kg / h will be passed into chlorination reaction continuously with fresh monochloroacetyl chloride Still, with the mass flow rate of 940.2kg / h, continuously extract the chlorination reaction liquid from the chlorination reaction kettle, this chlorination reaction liquid carries out phase separation in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com