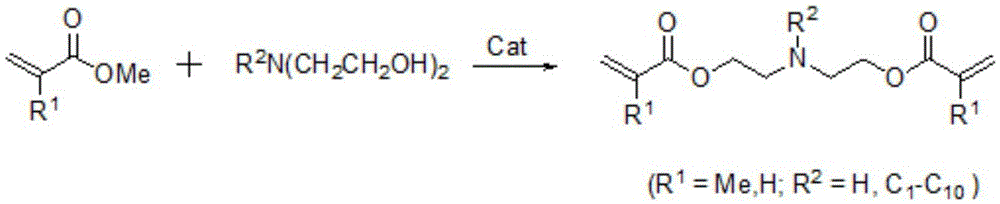Method for synthesizing diethanolamine acrylate compounds
A technology of diethanolamine acrylate and diethanolamine, which is applied in the field of synthesis of diethanolamine acrylate compounds, can solve the problems of environmental pollution, difficulty in recycling, and high catalyst price, and achieve high yield, mild preparation reaction conditions, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In the reactor that 5000ml is equipped with reactive distillation unit, add 1720g methyl acrylate, 500g toluene, nitroxide free radical piperidinol polymerization inhibitor 15.4g, the zinc bromide catalyst supported by 37.9gZSM-5 molecular sieve (loading capacity 14 %), turn on the stirring, raise the temperature to 70°C, add 595g of methyldiethanolamine dropwise, raise the temperature to reflux, and control the reflux ratio to 8:1. The reaction was detected by gas chromatography. After the reaction was completed, the catalyst was recovered by filtration and rectified under reduced pressure to obtain 911 g of methyldiethanolamine diacrylate with a purity greater than 99%.
Embodiment 2
[0019] In the reactor that 5000ml is equipped with reactive distillation unit, add 2000g methyl methacrylate, 500g heptane, hydroquinone monomethyl ether polymerization inhibitor 16.7g, the zinc bromide catalyst (loading capacity) of 34.4g gac support 22%), stirred, raised the temperature to 70°C, added dropwise 595g of methyldiethanolamine, raised the temperature to reflux, and controlled the reflux ratio to 9:1. The reaction was detected by gas chromatography. After the reaction was completed, the catalyst was recovered by filtration and rectified under reduced pressure to obtain 944 g of methyldiethanolamine dimethacrylate with a purity greater than 99%.
Embodiment 3
[0021] In the reactor that 5000ml is equipped with reactive distillation unit, add 2150g methyl acrylate, 550g cyclohexane, piperidine free radical phosphite triester polymerization inhibitor 17.3g, the zinc bromide catalyst ( Loading capacity 18%), stirring, heating up to 70°C, adding 905g of phenyldiethanolamine dropwise, heating up to reflux, and controlling the reflux ratio to 7:1. The reaction was detected by gas chromatography. After the reaction, the catalyst was recovered by filtration and rectified under reduced pressure to obtain 983 g of phenyldiethanolamine diacrylate with a purity greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com