Novel terpene compound with aldehyde group and preparation method and application thereof
A technology based on terpenoids and aldehyde groups, which can be used in medical preparations containing active ingredients, organic chemistry, drug combinations, etc., can solve problems that have not been seen before, and achieve low toxic and side effects, novel structure, and simple extraction and separation methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of formula (I) compound:
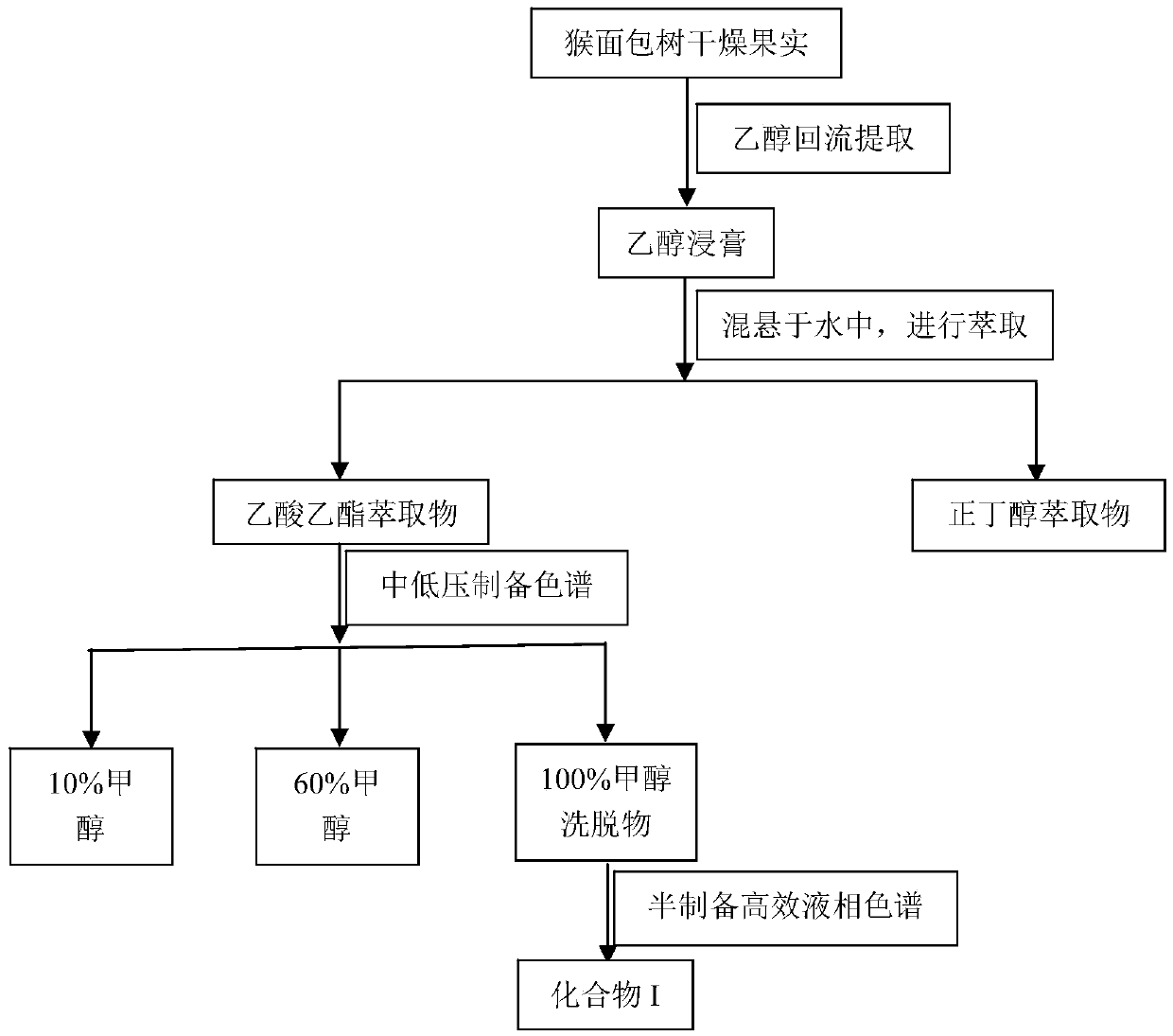
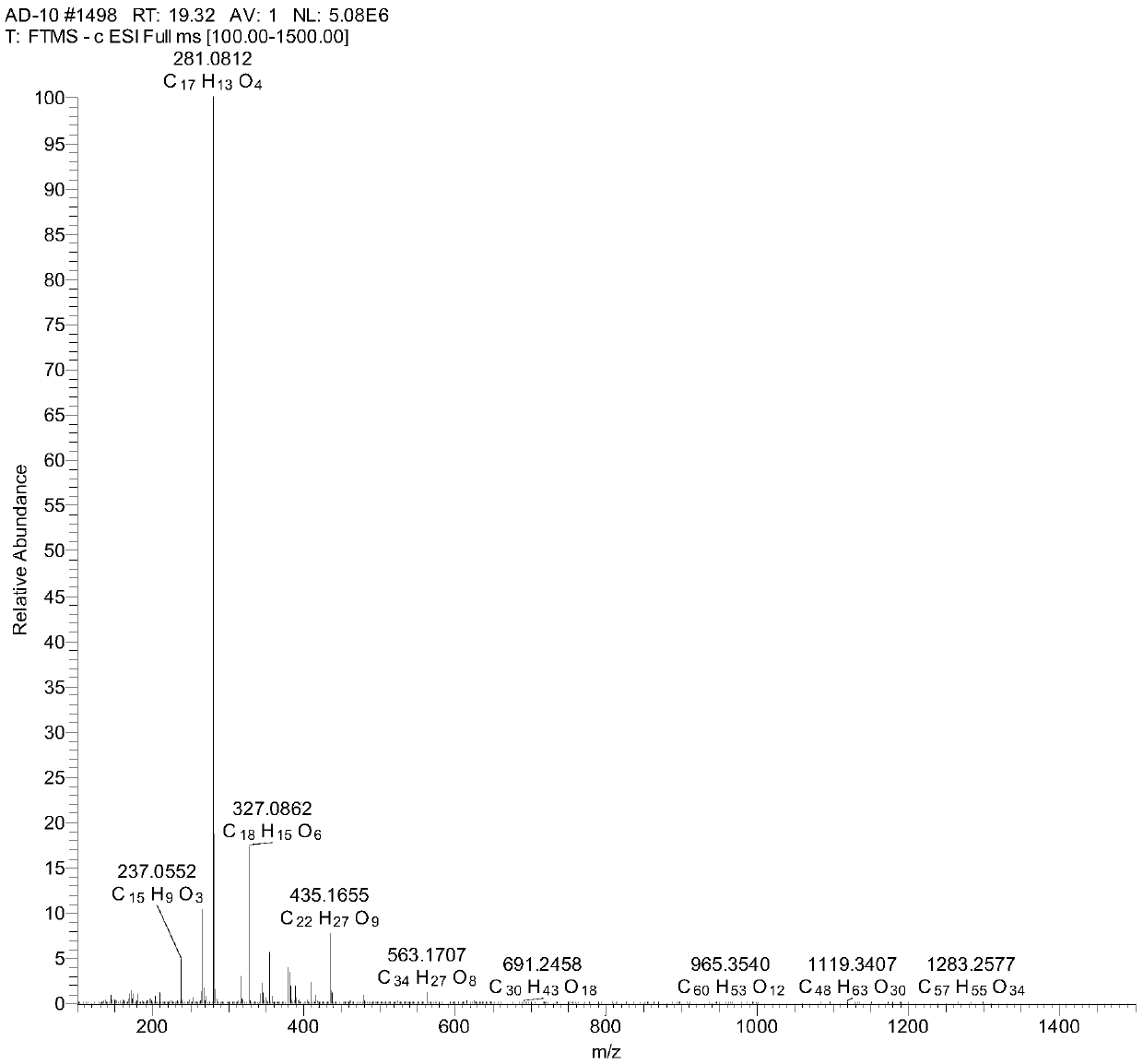
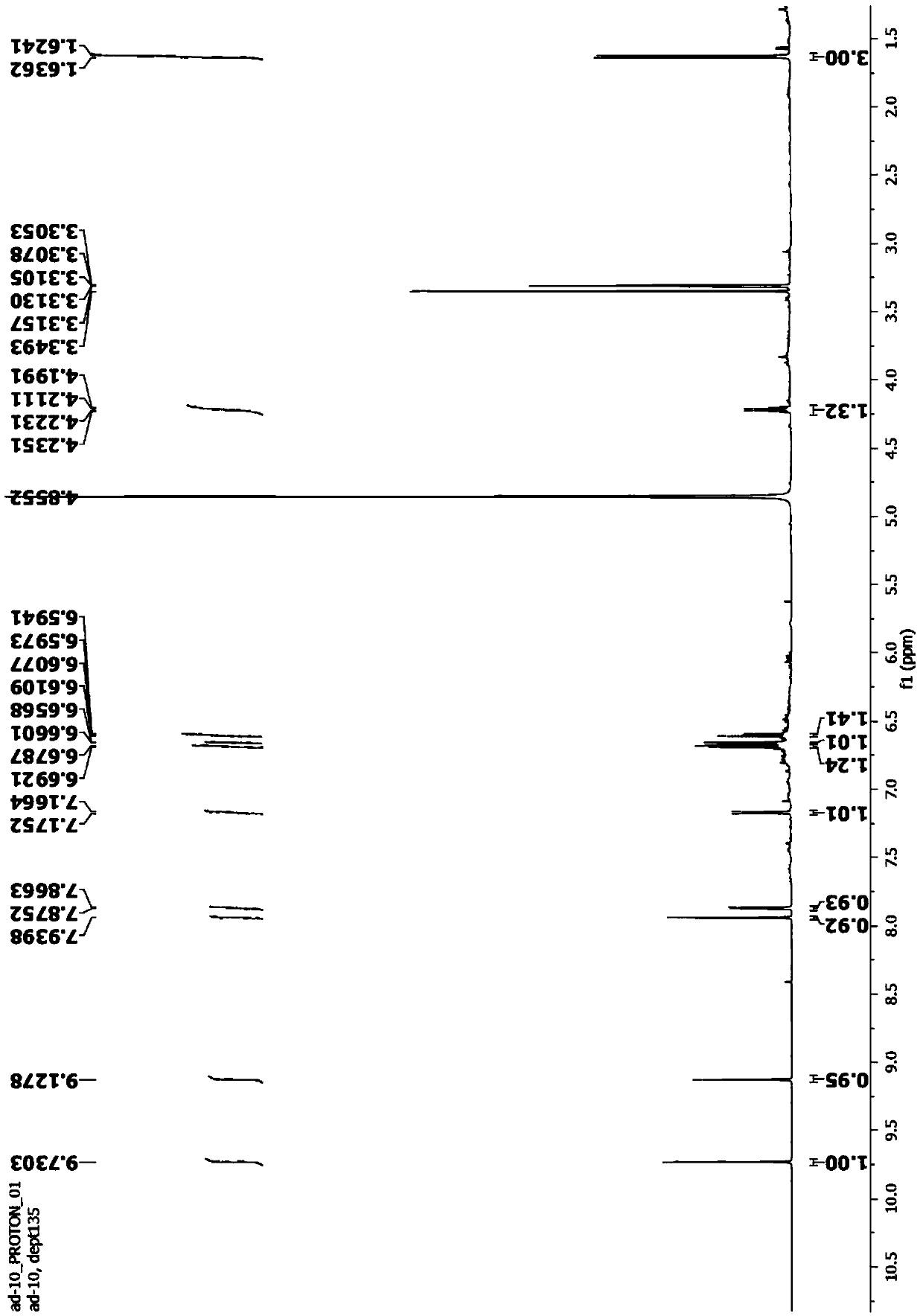
[0029] Using 0.8kg of dried baobab fruit as raw material, according to the solid-liquid ratio of 1:8 (kg / L), reflux extraction with 70% ethanol three times, each time for one hour, concentrate to obtain 75g of extract-like ethanol extract, and concentrate to obtain The ethanol extract was suspended in water, extracted with ethyl acetate (3 times, 750ml / time), and the extracted part of the extract extracted with ethyl acetate was separated by medium and low pressure preparative chromatography (C18 packing, 50um) and separated using methanol-water ( Methanol volume content is 10%, 60%, 100%) gradient elution successively, wherein the elution part of 100% methanol is separated by semi-preparative high-performance liquid chromatography (AgilentXDB-C18reversed-phasecolumn-5μm, 250×10mm) again, with Acetonitrile-water (30:70 by volume) was eluted to obtain the compound of formula I (10 mg).
[0030] The obtained compou...
Embodiment 2
[0038] Embodiment 2: Formula (I) compound is to the growth inhibitory experiment of human breast cancer MDA-MB-231 cell in vitro: MDA-MB-231 cell monolayer is inoculated in containing mass concentration 2% glutamine, 1.5% sodium bicarbonate , 10% fetal bovine serum in RPMI-1640 culture medium. And added 100 units / ml of penicillin and 100 μg / ml of streptomycin. At a temperature of 37°C, CO 2 Cultured in a cell culture incubator at a concentration of 5%. Cells in the logarithmic growth phase were prepared as 1 × 10 4 Inoculate the concentration of cell / mL on a 96-well plate, 0.1mL per well, and then add medium containing different concentrations into the wells, each concentration has 3 parallel groups, add the same amount of solvent to the control group, and place in a 37°C carbon dioxide incubator Culture in medium for 72h, then centrifuge (1000rpm, 20min), discard the supernatant, add 0.20mg / mL MTT serum-free medium to each well, continue to culture at 37°C for 3h, centrifu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com