Strong promoter and application of strong promoter to improving expression of nattokinase
A strong promoter and promoter technology, applied in the direction of enzymes, peptidases, hydrolytic enzymes, etc., can solve the problem that the production of nattokinase is insufficient to meet production needs, and achieve good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
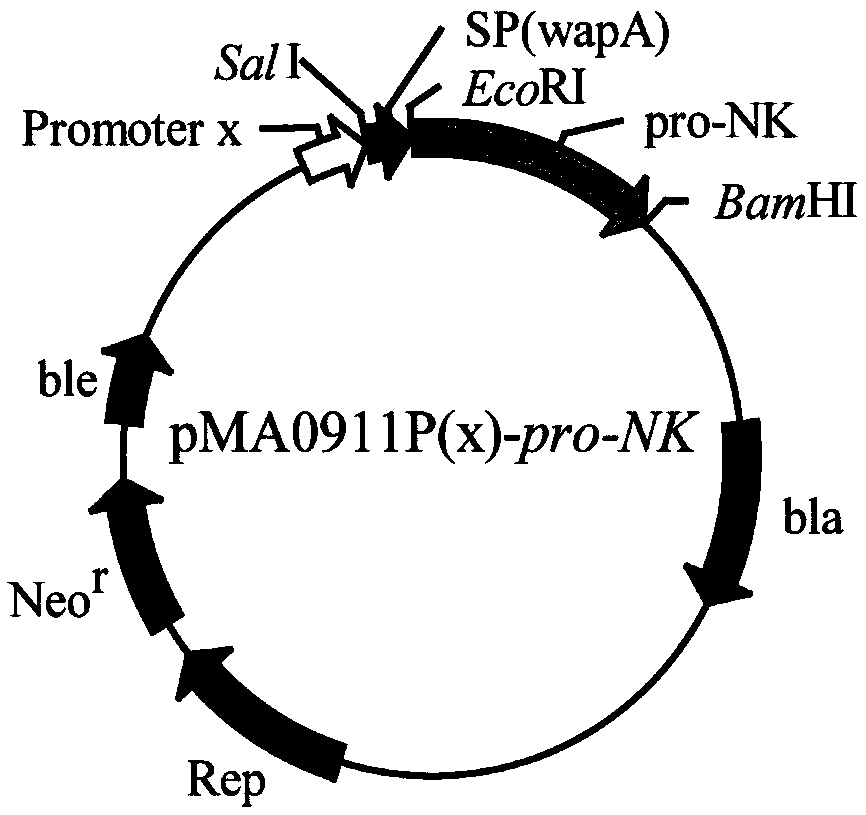
[0043] Example 1: pMA0911P HpaII -Construction of pro-NK vector
[0044] According to the nattokinase gene sequence (Genbank accession number: S51909.1) provided by Genbank, primers pro-NK-F / pro-NK-R were designed, and the genomic DNA of Bacillus natto (B.natto) was used as a template for amplification pro-nk gene, the gel electrophoresis band is about 1100bp, which is consistent with the size of the pro-nattokinase gene; (2) the PCR product pro-nk is purified, and after double digestion with EcoRI and BamHI, it is connected to On the Escherichia coli-Bacillus subtilis shuttle plasmid pMA0911-wapA double-digested with endonuclease, the recombinant expression plasmid pMA0911P was obtained by ligation HpaII-pro-NK, transform the plasmid into Escherichia coli JM109, and send it for sequencing after digestion and verification. The sequencing results show that the nattokinase gene was successfully inserted into the vector pMA0911-wapA; (3) extract the plasmid and transform it into...
Embodiment 2
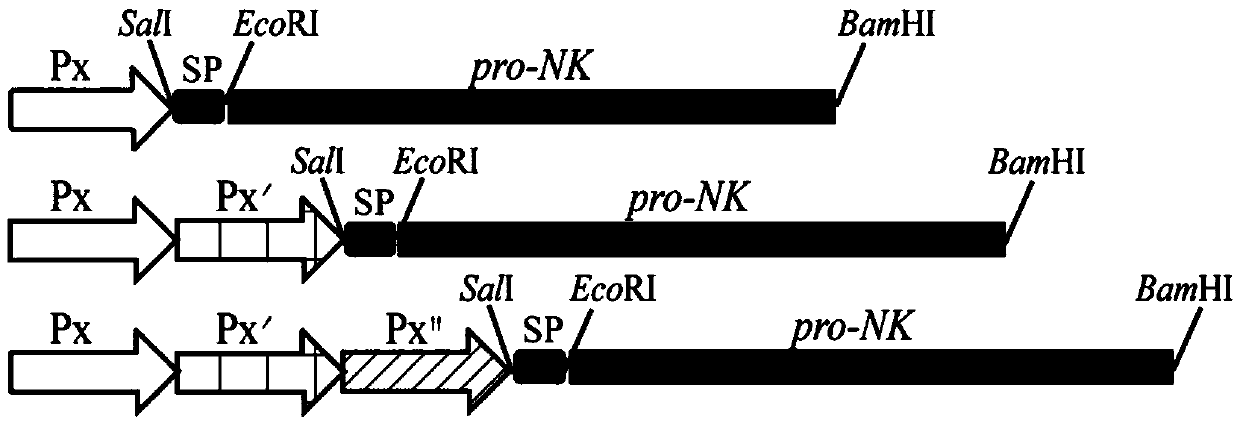
[0047] Embodiment 2: the design of strong promoter gene sequence
[0048] promoter P HpaII- P HpaII The design of the gene sequence:
[0049] (1) According to P HpaII Promoter gene sequence design primer P HpaII- P HpaII -F / P HpaII- P HpaII -R, to pMA0911P HpaII -Pro-NK plasmid was used as a template for PCR, and the resulting PCR product was used as a primer, pMA0911P HpaII -Pro-NK plasmid was used as a template for large primer PCR, and the obtained PCR product was treated with DpnⅠ and then transformed into Escherichia coli JM109 for plasmid amplification. After coating a plate containing resistance, it was cultured at 37°C, and a single colony was picked and transferred to a plate containing Resistant liquid 2×YT medium culture;
[0050] (2) DNA sequencing, the sequencing results show that the target gene fragment is successfully inserted into the original vector P HpaII Behind the gene sequence, a new promoter P is formed HpaII -P HpaII (The nucleotide sequenc...
Embodiment 3
[0052] Embodiment 3: fermentative synthesis of recombinant nattokinase
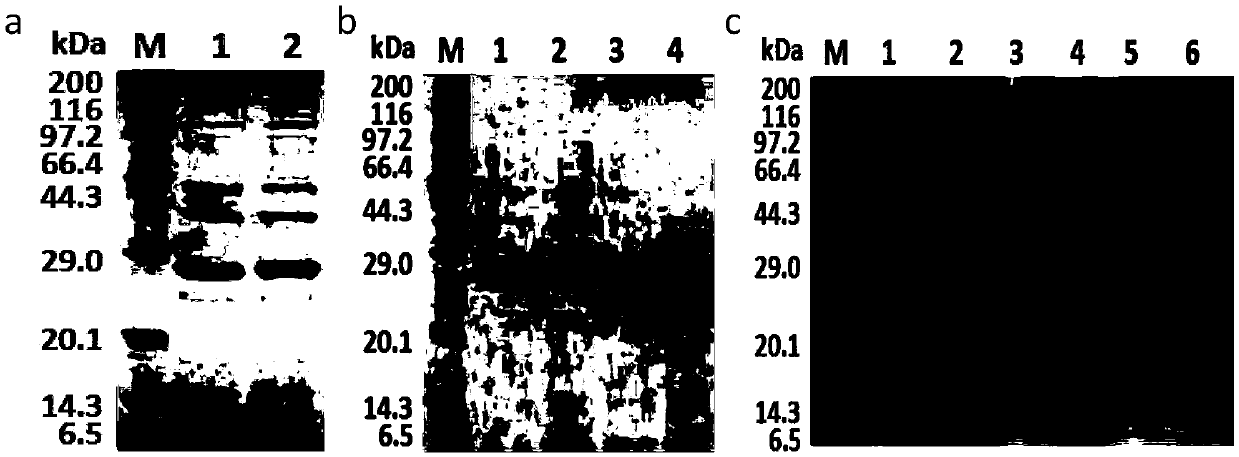
[0053] Construction of recombinant bacteria: transfer the recombinant plasmid with the correct sequence obtained in Example 2 into Bacillus subtilis WB800, and after standing overnight at 37°C, pick a single colony and place it in 10mL 2×YT seed medium at 37°C Cultivate; transfer to a 250mL Erlenmeyer flask containing 30mL TB medium according to the inoculation amount of 1-5%, 200r / min, temperature 37°C, and cultivate for 48 hours. The fermentation supernatant was taken for SDS-PAGE protein electrophoresis ( image 3 ).
[0054] Such as image 3 As shown, the electrophoresis band of about 28kDa was obtained, indicating that the recombinant Bacillus subtilis successfully secreted and expressed nattokinase. The fibrinolytic activity of each recombinant nattokinase was detected by ultraviolet spectrophotometer ( Figure 4 ), the specific results are shown in Table 2. The results showed that the expressi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com