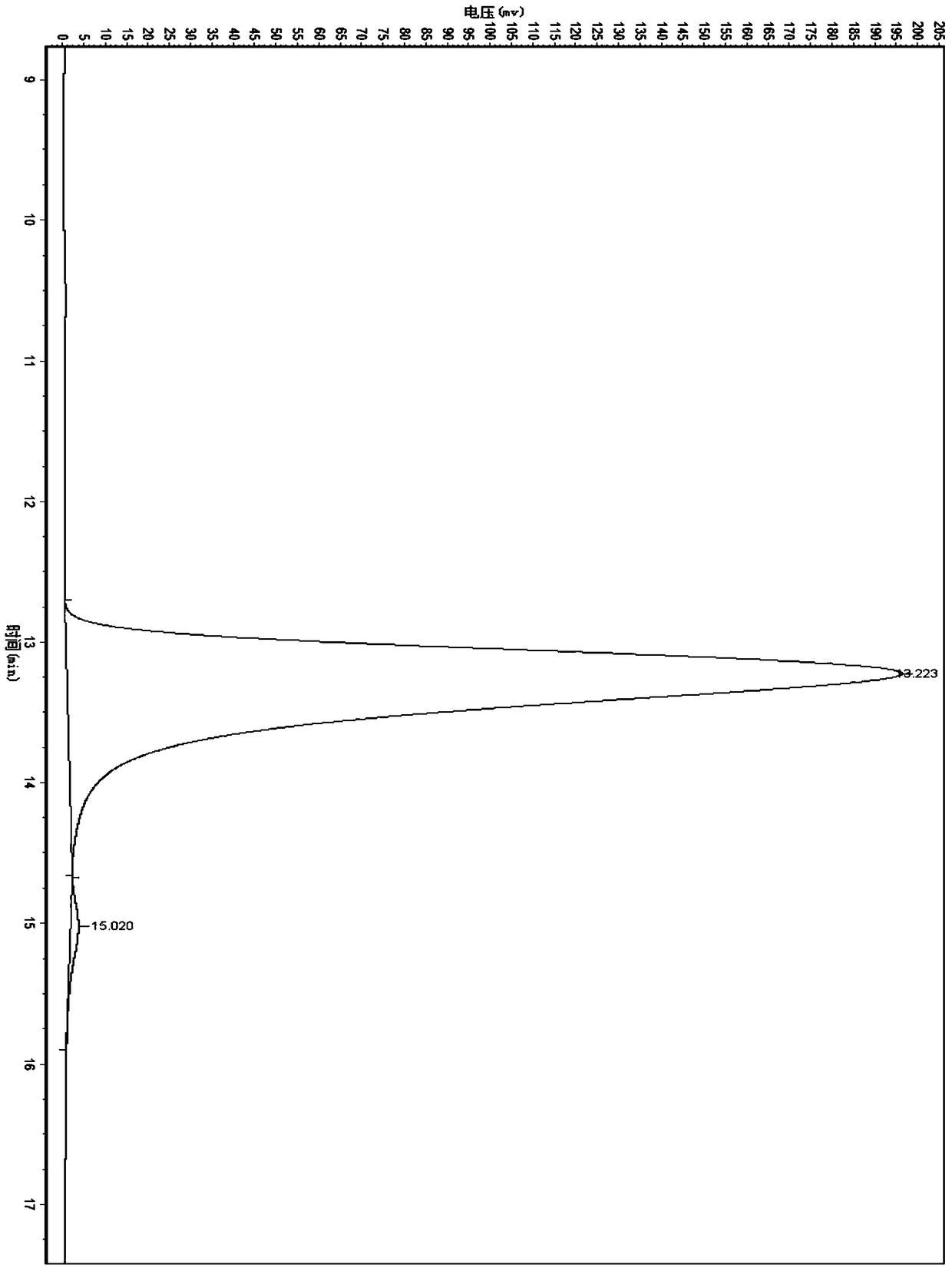
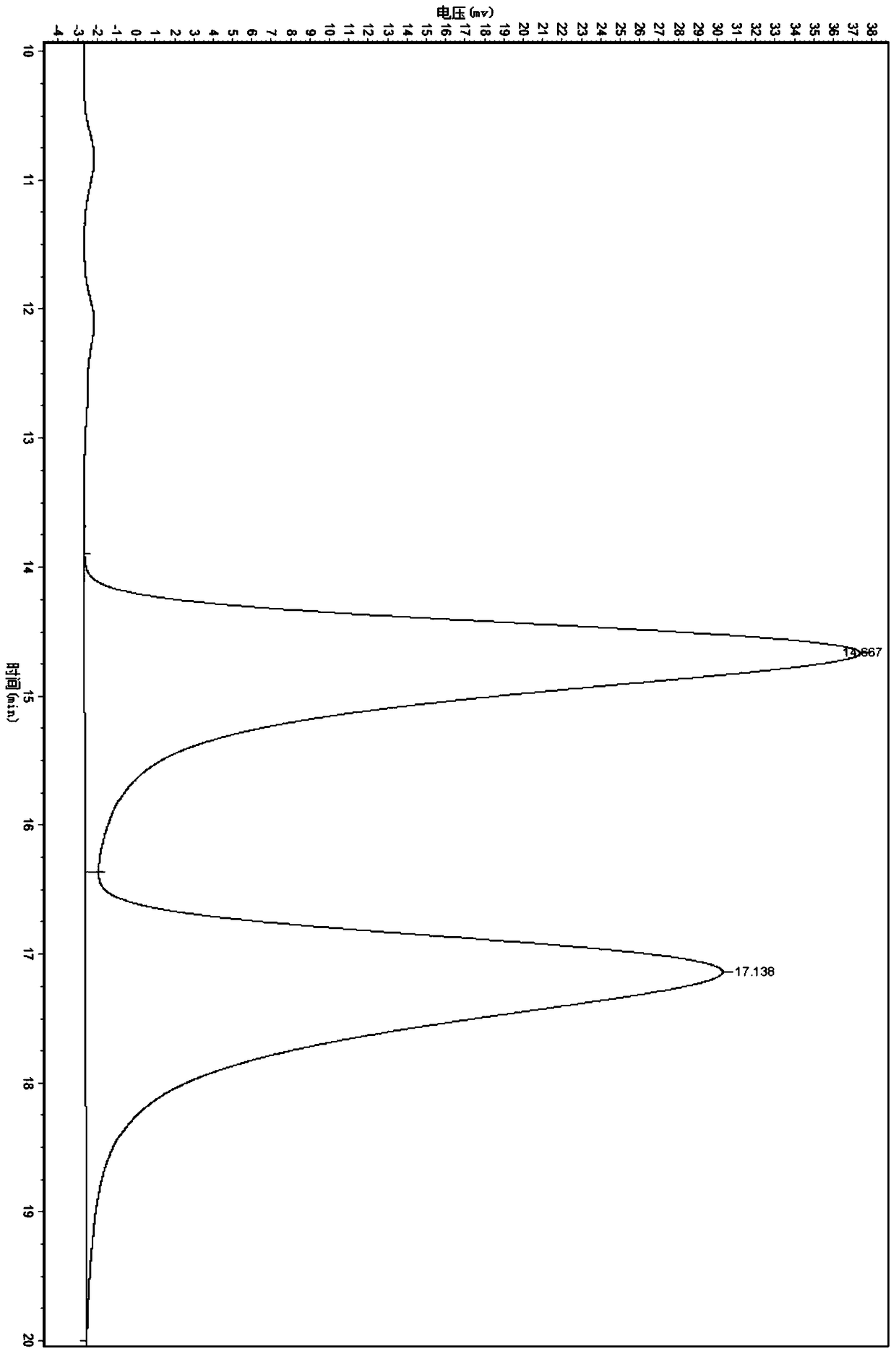
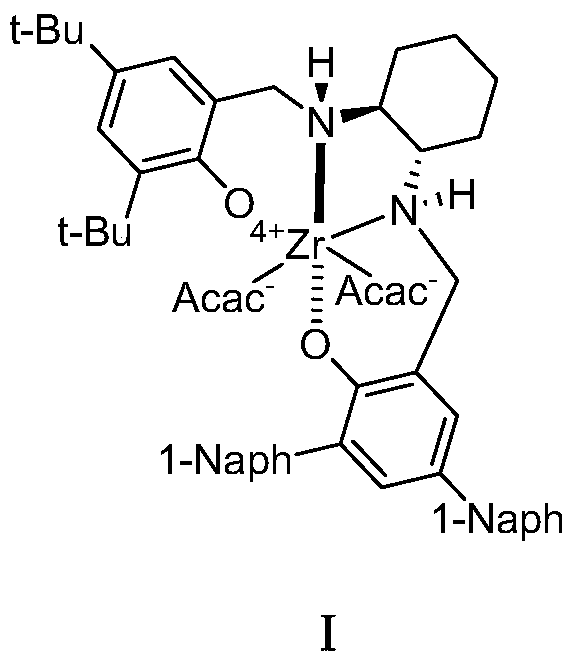
A kind of zirconium catalyst and the method for preparing chiral α-hydroxy-β-keto ester compound
A technology of compound and ketoester, which is applied in the field of asymmetric catalytic synthesis, can solve the problems of limited application and complex structure, and achieve the effects of low price, simple operation and good enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of (1S, 2S)-1,2-bis((4,6-diphenyl)phenol-2-methylamino)-cyclohexane (formula IIa, R 5 , R 6 for phenyl)
[0044] Weigh 2.28g (20mmol) of (1S,2S)-1,2-cyclohexanediamine and dissolve it in 100mL of methanol, and dissolve 10.96g (40mmol) of 2-hydroxy-3,5-diphenyl-benzaldehyde in 50mL of THF After slowly adding dropwise to the system at room temperature, the system was added to reflux for 3 hours, and the system turned into a yellow solution. Subsequently, the system was cooled to room temperature, and 3.08 g (80 mmol) of sodium borohydride was added in portions, followed by reaction at room temperature for 1 hour. Add 50 mL of water and 100 mL of dichloromethane to the system, separate the layers, extract the aqueous layer with 30 mL of dichloromethane x 2, wash the combined dichloromethane with water and 50 mL of saturated brine, and dry over anhydrous sodium sulfate. After filtration, the solvent was removed by rotary evaporation under reduced pr...
Embodiment 2
[0045] Example 2 Preparation of (1S, 2S)-1,2-bis((4,6-bis(1-naphthyl))phenol-2-methylamino)-cyclohexane (formula IIa, R 5 , R 6 for 1-naphthyl)
[0046] Weigh 1.71g (15mmol) of (1S,2S)-1,2-cyclohexanediamine and dissolve it in 100mL of methanol, and dissolve 11.22g (30mmol) of 2-hydroxy-3,5-diphenyl-benzaldehyde in 50mL of THF After slowly adding dropwise to the system at room temperature, the system was added to reflux for 3 hours, and the system turned into a yellow solution. Subsequently, the system was cooled to room temperature, and 2.31 g (60 mmol) of sodium borohydride was added in portions, followed by reaction at room temperature for 1 hour. Add 50 mL of water and 100 mL of dichloromethane to the system, separate the layers, extract the aqueous layer with 30 mL of dichloromethane x 2, wash the combined dichloromethane with water and 50 mL of saturated brine, and dry over anhydrous sodium sulfate. After filtration, the solvent was removed by rotary evaporation under...
Embodiment 3
[0047] Example 3 Preparation of (1S,2S)-2-((3,5-di-tert-butyl-2-hydroxybenzylidene)amino)cyclohexane-1-amine hydrochloride
[0048] Weigh 5.7g (50mmol) of (1S,2S)-1,2-cyclohexanediamine and dissolve it in 100mL of methanol, measure 4.17mL (50mmol) of concentrated hydrochloric acid with a pipette, and slowly add it dropwise in an ice bath, Stirring was continued for 30 minutes after the dropwise addition was complete. Subsequently, weigh 11.7g (50mmol) of 3,5-di-tert-butyl-2-hydroxy-benzaldehyde and dissolve it in 50mLTHF, and slowly add it dropwise in an ice bath (not less than 50 minutes), and then the system rises to room temperature The reaction was stopped after 35 minutes of reaction. The solvent was removed under reduced pressure, 50 mL of methanol was added to the residue, and suction filtration was performed under reduced pressure. After drying in vacuo, 17.9 g of a yellow solid was obtained, with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com