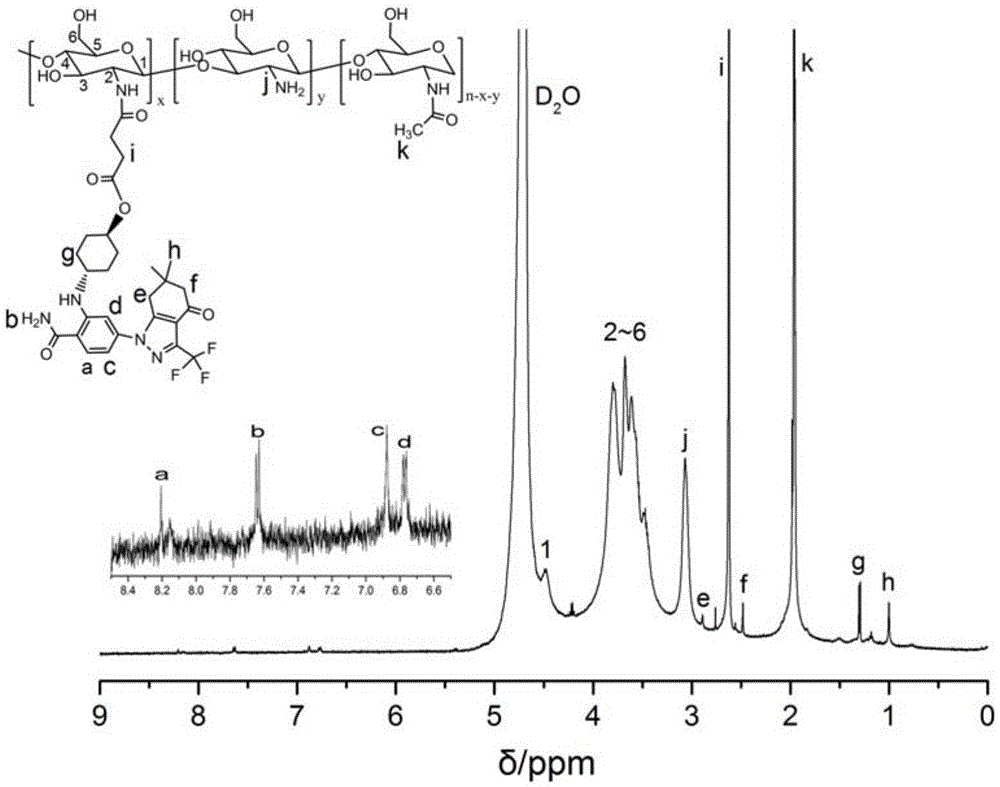
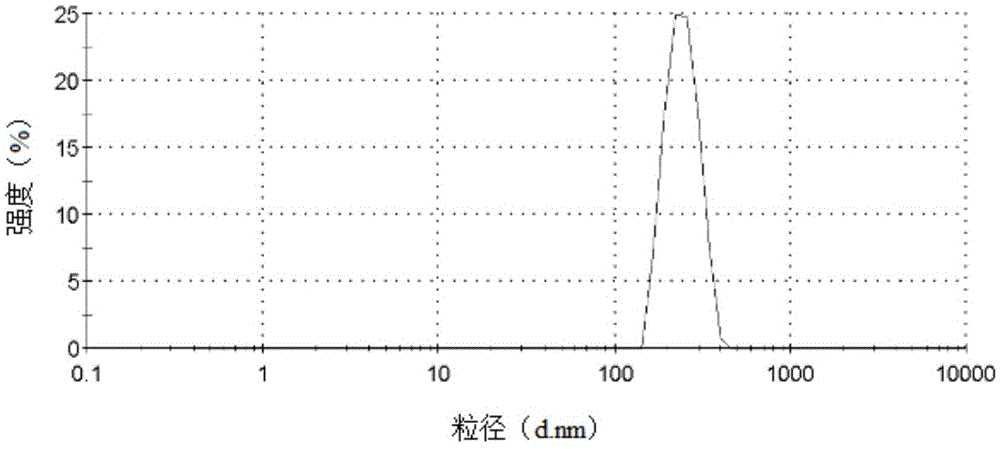
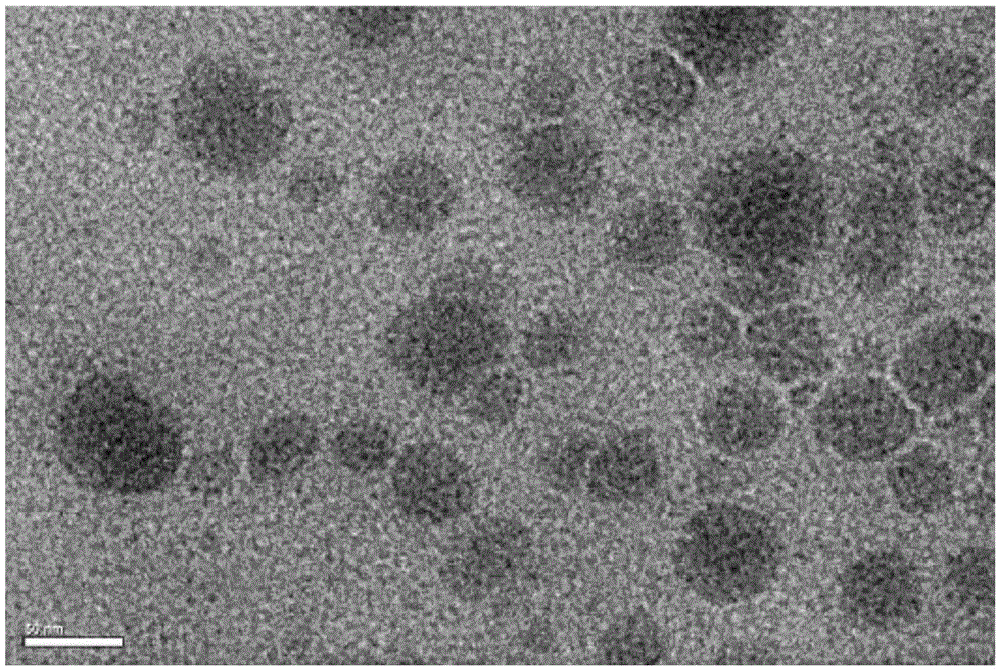
Core-shell structure nanoparticles for reduction/enzyme/pH multi-responsive drug release
A core-shell structure and nanoparticle technology, applied in antitumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as non-degradability and high cytotoxicity, and achieve easy drug release, high drug loading, and improved blood stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] (1) Preparation of core layer chitosan-based highly drug-loaded nanomicelles
[0078] ① Dissolve 150mg of SNX2112 and 40mg of succinic anhydride in 10mL of DMSO; add 0.1mL of triethylamine to the above solution, and conduct an esterification reaction at room temperature for 72 hours; rotate the solution after the reaction to remove the solvent and triethylamine under reduced pressure, and then Washing with acetone to remove the residual unreacted drug, then washing with water to remove the residual unreacted succinic anhydride, and freeze-drying to obtain the succinic anhydride modified drug;
[0079] ② Dissolve 20 mg of the succinic anhydride modified drug prepared in step ① in 20 mL of DMSO, and add 0.07 mmol of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride to the solution (EDC·HCl) and 0.07mmol N-hydroxysuccinimide (NHS), activation reaction 0.5h; take 40mgCs dissolved in 6mL2% (W / W) acetic acid solution, then add to 34mL MES buffer solution of pH=5.6 a...
Embodiment 2
[0088] (1) Preparation of core layer chitosan-based highly drug-loaded nanomicelles
[0089] ①Dissolve 50mg of paclitaxel and 20mg of succinic anhydride in 10mL of DMSO; add 0.5mL of triethylamine to the above solution, and perform esterification reaction at room temperature for 24 hours; spin evaporate the solution under reduced pressure to remove the solvent and triethylamine Amine, then wash with acetone to remove residual unreacted drug, then wash with water to remove residual unreacted succinic anhydride, obtain succinic anhydride modified drug after freeze-drying;
[0090] ② Dissolve 20 mg of the succinic anhydride modified drug prepared in step ① in 20 mL of DMSO, and add 0.7 mmol of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride to the solution (EDC·HCl) and 0.07mmol N-hydroxysuccinimide (NHS), activation reaction for 24h; take 100mg of Cs dissolved in 3mL of 2% (W / W) acetic acid solution, and then add to 17mL of MES buffer solution mixed solvent with pH=5...
Embodiment 3
[0099] (1) Preparation of core layer chitosan-based highly drug-loaded nanomicelles
[0100]①Dissolve 100mg of doxorubicin and 30mg of succinic anhydride in 10mL of DMSO; add 0.25mL of triethylamine to the above solution, and perform esterification reaction at room temperature for 48 hours; spin evaporate the solution after the reaction to remove the solvent and triethylamine Amine, then wash with acetone to remove residual unreacted drug, then wash with water to remove residual unreacted succinic anhydride, obtain succinic anhydride modified drug after freeze-drying;
[0101] ② Dissolve 50 mg of the succinic anhydride modified drug prepared in step ① in 20 mL of DMSO, and add 0.35 mmol of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride to the solution (EDC·HCl) and 0.07mmol N-hydroxysuccinimide (NHS), activation reaction for 12h; take 50mg of Cs dissolved in 3mL of 2% (W / W) acetic acid solution, then add to 17mL of MES buffer solution with pH=5.6 and sonicate Aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com