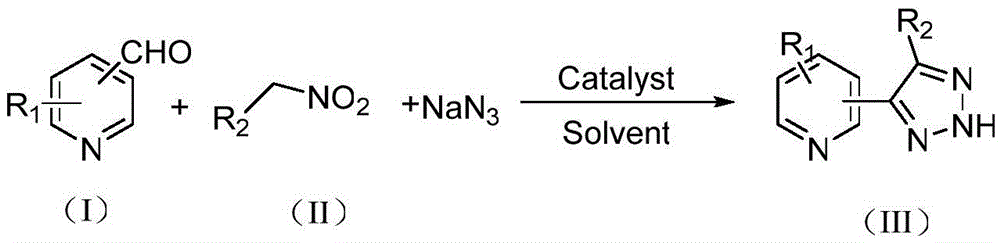
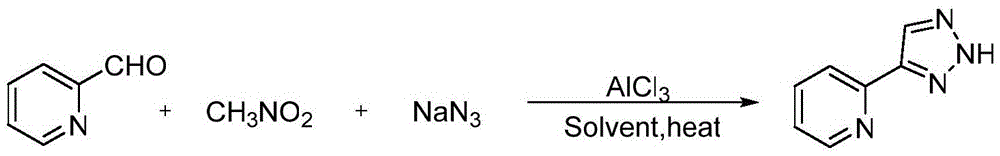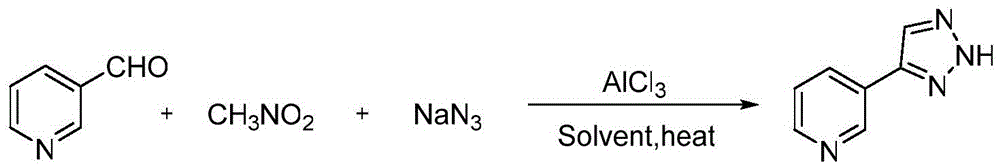Synthetic method of NH-1,2,3-triazole bipyridine compounds
A technology of triazole bipyridine and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of many steps, difficult synthesis, complicated operation and the like, and achieves the effects of mild reaction conditions, shortened synthesis route and wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Synthesis of 4-(2-pyridyl)-2H-1,2,3-triazole:
[0020] The reaction formula is:
[0021]
[0022] The specific steps are: add 0.33mmol 2-pyridinecarbaldehyde, 0.50mmol nitromethane, 0.39mmol sodium azide, 0.04mmol AlCl to a 50mL round bottom flask 3 , 2mLDMSO, magnetically stirred and reacted at 80°C for 12 hours, then extracted the reaction solution with ethyl acetate, washed the organic layer with saturated brine, dried over anhydrous sodium sulfate, and evaporated the solvent under reduced pressure to obtain the crude product. Ethyl acetate / petroleum ether=1:3 (V / V) was used as the eluent for column separation and purification to obtain the desired product, which was a white solid with a yield of 86%.
[0023] The proton nuclear magnetic spectrogram result of gained product is: 1 HNMR (600MHz, CDCl 3 ): δ7.97(s, 1H), 7.51(d, J=1.2Hz, 1H), 6.85(d, J=3.3Hz, 1H), 6.51(m, 1H). The result of NMR spectrum is: 13 CNMR (150MHz, CDCl 3 ): δ145.3, 142.8, 111.6, 107.8....
Embodiment 2
[0025] Synthesis of 4-(3-pyridyl)-2H-1,2,3-triazole:
[0026] The reaction formula is:
[0027]
[0028] The specific steps are: add 0.33mmol 3-pyridinecarbaldehyde, 0.50mmol nitromethane, 0.39mmol sodium azide, 0.04mmol AlCl to a 50mL round bottom flask 3 , 2mLDMSO, magnetically stirred and reacted at 80°C for 12 hours, then extracted the reaction solution with ethyl acetate, washed the organic layer with saturated brine, dried over anhydrous sodium sulfate, and evaporated the solvent under reduced pressure to obtain the crude product. Ethyl acetate / petroleum ether=1:3 (V / V) was used as the eluent for column separation and purification to obtain the desired product, which was a white solid with a yield of 91%.
[0029] The proton nuclear magnetic spectrogram result of gained product is: 1 HNMR (500MHz, CDCl 3 -DMSO-d6)d=9.06(s,1H),8.57(s,1H),8.15-8.14(d,J=2.0Hz,1H),7.99(d,J=1.8Hz,1H),7.41-7.38 (m,1H).
Embodiment 3
[0031] Synthesis of 4-(4-pyridyl)-2H-1,2,3-triazole:
[0032] The reaction formula is:
[0033]
[0034] The specific steps are: add 0.33mmol 4-pyridinecarbaldehyde, 0.50mmol nitromethane, 0.39mmol sodium azide, 0.04mmol AlCl to a 50mL round bottom flask 3 , 2mLDMSO, magnetically stirred at 80°C for 12 hours, then extracted the reaction solution with ethyl acetate, washed the organic layer with saturated brine, dried over anhydrous sodium sulfate, and evaporated the solvent under reduced pressure to obtain the crude product. Ethyl acetate / petroleum ether=1:3 (V / V) was used as the eluent for column separation and purification to obtain the desired product, which was a white solid with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com