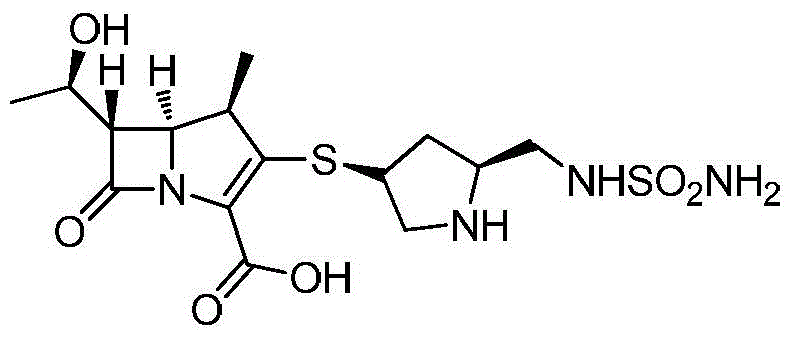
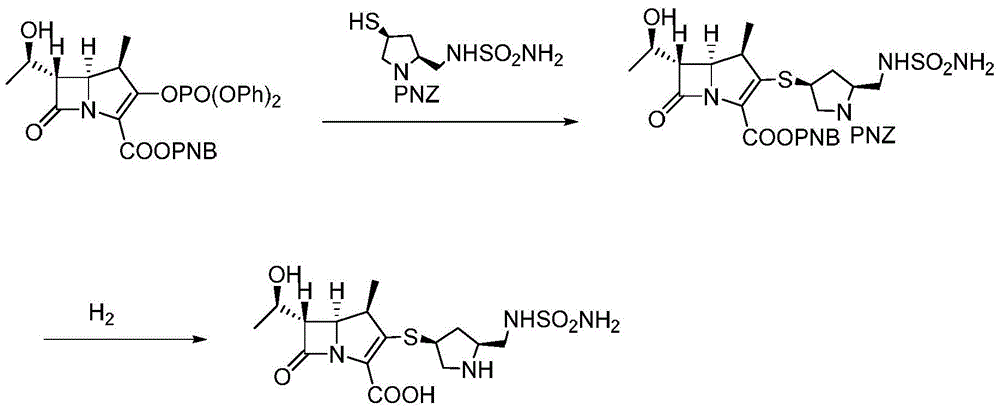
A kind of preparation method of high-purity doripenem
A technology of doripenem and high purity, applied in the field of preparation of high-purity doripenem, can solve the problems of difficult product purification, many by-products, low reaction yield and the like, and achieves short reaction time, improved yield, The effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method of doripenem, the preparation method comprising the following steps:
[0031]1) In the presence of copper nitrate and triethylamine, the carbapenem bicyclic nucleus (60.8g, 0.1mol) was mixed with (2S,4S)-1-p-nitrobenzyloxycarbonyl-4-thio-2- (N-sulfamoylamino)methylpyrrolidine was contacted in a mixed solvent of 800mL water and 1,4-dioxane (the volume ratio of water and 1,4-dioxane was 1:15) , the conditions of the contact reaction include first reacting at 15°C until the reaction of the carbapenem bicyclic nucleus is completed, then cooling down to -10°C to continue the reaction for 2 hours, and then raising the temperature to 10°C for 2 hours. After the reaction, add water and ethyl acetate to stir, let stand to separate layers, concentrate the ethyl acetate layer, then recrystallize from the mixed solvent of dichloromethane and sherwood oil (the volume ratio of dichloromethane and sherwood oil is 1:8) Get (1R,5S,6S)-2-[(3S,5S)-1-benzyl p-nitrofor...
Embodiment 2
[0034] A preparation method of doripenem, the preparation method comprising the following steps:
[0035] 1) In the presence of copper nitrate and triethylamine, the carbapenem bicyclic nucleus (60.8g, 0.1mol) was mixed with (2S,4S)-1-p-nitrobenzyloxycarbonyl-4-thio-2- (N-sulfamoylamino) methylpyrrolidine is contacted in a mixed solvent of 800ml water and 1,4-dioxane (the volume ratio of water and 1,4-dioxane is 1:15) , the conditions of the contact reaction include first reacting at 13°C until the reaction of the carbapenem bicyclic nucleus is completed, then cooling down to -15°C to continue the reaction for 1 hour, and then raising the temperature to 15°C for 2 hours. After the reaction, add water and ethyl acetate to stir, let stand to separate layers, concentrate the ethyl acetate layer, then recrystallize from the mixed solvent of dichloromethane and sherwood oil (the volume ratio of dichloromethane and sherwood oil is 1:6) Get (1R,5S,6S)-2-[(3S,5S)-1-benzyl p-nitroform...
Embodiment 3
[0038] A preparation method of doripenem, the preparation method comprising the following steps:
[0039] 1) In the presence of copper chloride and triethylamine, the carbapenem bicyclic nucleus (60.8g, 0.1mol) was mixed with (2S,4S)-1-p-nitrobenzyloxycarbonyl-4-thio-2 -(N-sulfamoylamino)methylpyrrolidine is contacted in a mixed solvent of 800ml water and 1,4-dioxane (the volume ratio of water and 1,4-dioxane is 1:10) Reaction, the conditions of the contact reaction include first reacting at 10°C until the reaction of the carbapenem bicyclic nucleus is completed, then cooling down to -10°C to continue the reaction for 2 hours, and then raising the temperature to 15°C for 3 hours. After the reaction, add water and ethyl acetate to stir, let stand to separate layers, concentrate the ethyl acetate layer, then recrystallize from the mixed solvent of dichloromethane and sherwood oil (the volume ratio of dichloromethane and sherwood oil is 1:5) Get (1R,5S,6S)-2-[(3S,5S)-1-benzyl p-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com