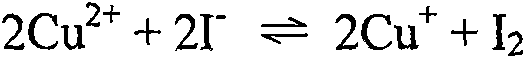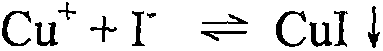Determination of trace arsenic in high-copper-content sample with atomic fluorescence spectrometry
An atomic fluorescence, trace arsenic technology, applied in the field of analysis and detection, can solve the problems of low measurement results, low data reliability, and difficult to clean, and achieve the effect of simplifying experiments, simple spectral lines, and fast and accurate detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]Taking the food additive copper sulfate as an example, the samples are processed with reference to the "National Food Safety Standard Food Additive Copper Sulfate", marked as T1 and T2; T7 and T8; while comparing samples only treated with potassium iodide, marked as T3 and T4, and only treated with potassium thiocyanate, marked as T5 and T6. After the treatment, the sample solution was reacted with thiourea-ascorbic acid mixed solution and potassium hydroxide-potassium borohydride solution to observe the reaction phenomenon. The specific experimental process is as follows:
[0048] Weigh 5.0 g of copper sulfate pentahydrate, a total of 8 parts, respectively marked as T-1, T-2, T-3, T-4, T-5, T-6, T-7 and T-8.
[0049] Add about 20mL of water to dissolve each of T-1 and T-2, transfer to a 100mL volumetric flask, dilute to the mark with water, shake well and set aside.
[0050] Add about 10mL of water to dissolve each of T-3 and T-4, add 20mL of potassium iodide solution ...
Embodiment 2
[0058] The trace arsenic in copper sulfate pentahydrate provided by a reagent factory was tested, and at the same time, standard addition recovery experiments and precision experiments were performed.
[0059] Weigh 5.0g of the sample, a total of 20 parts, marked as S-1, S-2, A-1, A-2, A-3, A-4, A-5, A-6, B-1, B-2, B-3, B-4, B-5, B-6, C-1, C-2, C-3, C-4, C-5 and C-6. After adding water to dissolve, add 20mL of potassium iodide solution with a mass fraction of 100%, stir well with a glass rod, add 10mL of potassium thiocyanate solution with a mass fraction of 100%, continue to stir evenly with a glass rod, and then filter to a capacity of 100mL In the bottle, adjust the volume to the mark with water, and shake well (note: here the reaction mass ratio of the initial copper ion to the added iodide ion and thiocyanate ion is 1:4:2).
[0060] Take 10mL of the above solution into a 50mL volumetric flask, add 2.5mL hydrochloric acid, 2.5mL thiourea-ascorbic acid mixed solution (the ...
Embodiment 3
[0067] Determination of trace arsenic in food additive copper sulfate. Weigh 5.0g of food additive copper sulfate into a 150mL beaker, add an appropriate amount of water to dissolve, add 20mL of potassium iodide solution with a mass fraction of 100%, stir well with a glass rod, then add 10mL of a 100% potassium thiocyanate solution Continue to stir evenly with a glass rod, then filter into a 100mL volumetric flask (note: the mass ratio of the initial copper ion to the added iodide ion and thiocyanate ion here is 1:4:2), add 5mL hydrochloric acid, 5mL sulfur Urea-ascorbic acid mixed solution (the mass fraction of thiourea is 10%, the mass fraction of ascorbic acid is 10%), the volume is made up to the mark with water, shake well and then stand still for 30min, to be measured. Do the same blank test and standard recovery test. Prepare arsenic standard solution working curves with concentrations of 1 μg / L, 2 μg / L, 5 μg / L, 8 μg / L and 10 μg / L respectively. The fluorescence intens...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com