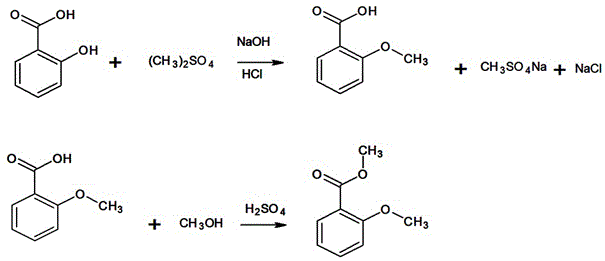
Synthesis method of methyl o-anisate
A technology of methyl o-methoxybenzoate and salicylic acid, which is applied in the field of organic compound synthesis, can solve problems such as high power consumption, slow decomposition speed, restrict large-scale production, etc., and achieves reduction of reaction cycle, simplified processing difficulty, The effect of shortening the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
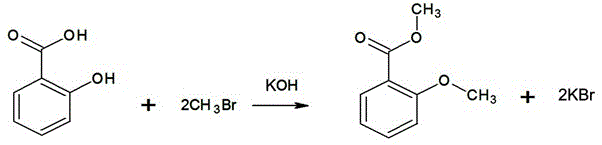
[0018] like figure 2 In the synthetic route shown, 69g (0.50mol) of salicylic acid, 138ml of water, 70g (1.25mol) of potassium hydroxide will be added to the reaction flask equipped with an exhaust gas absorption device, and the stirring speed will be adjusted to 100 rpm. Cool the material to 10°C, start to feed methyl bromide slowly, control the speed of feeding methyl bromide to ensure that no methyl bromide gas overflows, and control the temperature not to exceed 20°C, stop feeding methyl bromide when the methyl bromide reaches 95g (1.0mol), let stand, take For the lower water layer sample, add hydrochloric acid to adjust the pH to 3~4, extract with ethyl acetate, spot the extract (developer ethyl acetate:petroleum ether=30:70), observe only one product spot (no raw materials and intermediate products) The reaction is over, otherwise, continue to feed 1.45g (0.015mol) of methyl bromide until the reaction is complete. In this test, add 3 times to complete the reaction. Sto...
Embodiment 2
[0021] like figure 2 In the synthetic route shown, 69g (0.50mol) of salicylic acid, 150ml of water, 56g (1.00mol) of potassium hydroxide will be added to the reaction flask equipped with an exhaust gas absorption device, and the stirring speed will be adjusted to 80 rpm. Cool the material to 15°C, start to feed methyl bromide slowly, control the speed of feeding methyl bromide to ensure that no methyl bromide gas overflows, and at the same time control the temperature not to exceed 20°C, when the methyl bromide reaches 95g (1.0mol), suspend the feeding of methyl bromide, stand still, take For the lower water layer sample, add hydrochloric acid to adjust the pH to 3~4, extract with ethyl acetate, spot the extract (developer ethyl acetate:petroleum ether=30:70), observe only one product spot (no raw materials and intermediate products) reaction. Stop stirring, let stand to separate the lower layer of waste water, waste water is concentrated under reduced pressure and evaporate...
Embodiment 3
[0024] like figure 2 In the synthetic route shown, 69g (0.50mol) of salicylic acid, 140ml of water, 59g (1.05mol) of potassium hydroxide will be added to the reaction flask equipped with an exhaust gas absorption device, and the stirring speed will be adjusted to 120 rpm. Cool the material to 13°C, start slowly feeding methyl bromide, control the speed of feeding methyl bromide to ensure that no methyl bromide gas overflows, and at the same time control the temperature not to exceed 20°C, stop feeding methyl bromide when the methyl bromide reaches 95g (1.0mol), let stand, take For the lower water layer sample, add hydrochloric acid to adjust the pH to 3~4, extract with ethyl acetate, spot the extract (developer ethyl acetate:petroleum ether=30:70), observe only one product spot (no raw materials and intermediate products) The reaction is over, otherwise, continue to supplement 2.375g (0.025mol) of methyl bromide until the reaction is complete. In this test, add 4 times to com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com