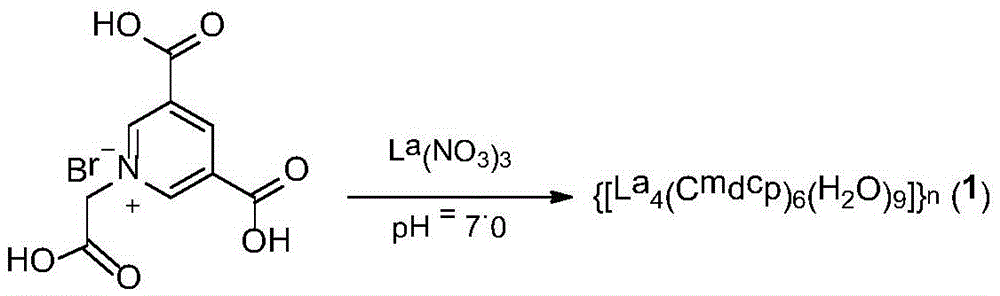Amphiprotic lanthanum carboxylate metal organic framework and synthesis method thereof, and application of amphiprotic lanthanum carboxylate metal organic framework
A metal-organic framework, amphoteric carboxylic acid technology, applied in biochemical equipment and methods, microbial determination/inspection, etc., can solve problems such as application research limitations, MOFs framework collapse, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
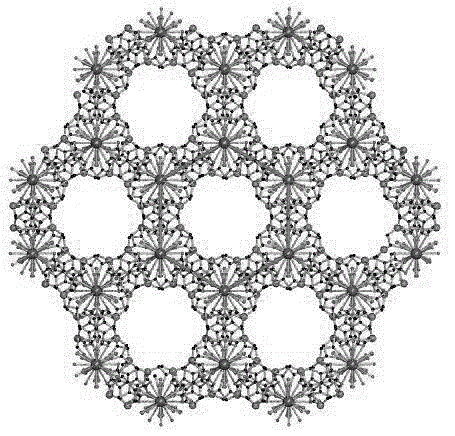
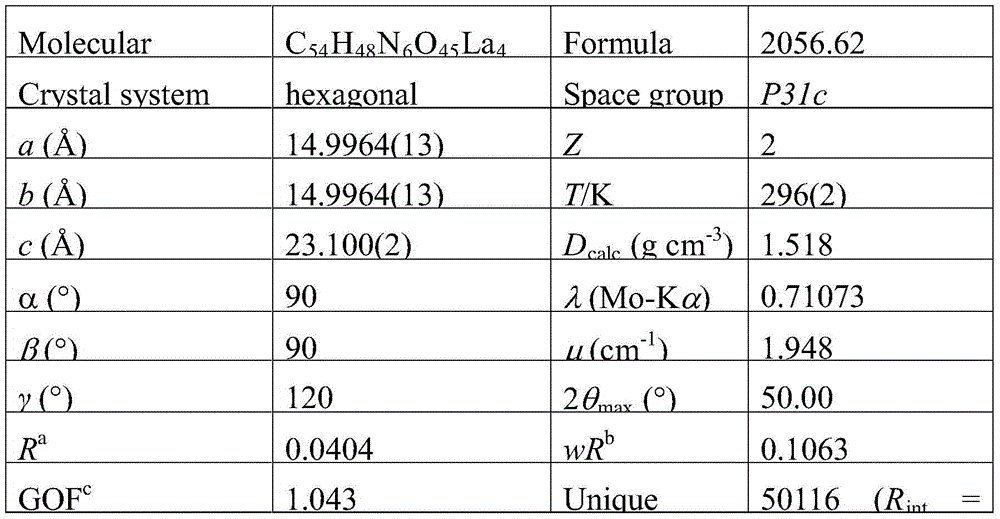
[0027] A kind of amphoteric lanthanum carboxylate metal organic framework, its chemical formula is {[La 4 (Cmdcp) 6 (H 2 O) 9 ]} n (1) (n represents the degree of aggregation, which is a natural number).
Embodiment 2
[0029] The preparation reaction formula of complex of the present invention is as follows:
[0030]
[0031] Specific steps are as follows:
[0032] a. Weigh the solid H according to the amount of the substance 3 CmdcpBr (91.5 mg, 0.3 mmol) was suspended in MeOH (5 mL) solution, and the pH was adjusted to 7.0 with 0.1 mM NaOH solution to obtain a clear solution.
[0033] b. Weigh the solid La(NO 3 ) 3 ·6H 2 O (86.6 mg, 0.2 mmol) was dissolved in MeOH solution (5 mL) to give a clear solution of the lanthanum salt.
[0034] c, the molar ratio of 2:3 La(NO 3 ) 3 ·6H 2 O solution was added dropwise to H 3 In the clear solution of CmdcpBr, the reaction was stirred at room temperature for 0.5h, and a large amount of white precipitates appeared in the reaction.
[0035] d, the precipitate is filtered, and the precipitate is washed with MeOH (5mL), and the white precipitate is washed with H 2 O (100 mL) was dissolved at 100 °C and filtered to obtain a clear solution.
[...
Embodiment 3
[0052] Compound {[La 4 (Cmdcp) 6 (H 2 O) 9 ]} n (1) Detect HIVds-DNA and SUDVRNA experiment (obtain compound in embodiment 1)
[0053] (1) Preparation of compound 1 solution
[0054] Compound 1 was dissolved in distilled water to prepare a solution with a concentration of 0.1mM, sealed and stored at room temperature; 2 ) to dissolve DNA and RNA to a concentration of 0.1 mM, seal and store in a refrigerator.
[0055] (2) Detection of HIVds-DNA and SUDVRNA experiments
[0056] Dilute the probe DNA solution with a buffer solution to a concentration of 50nM, take 2mL of the solution and put it into a cuvette with a capacity of 4mL. After adjusting the parameters of the fluorometer, test the initial fluorescence intensity of the solution. A certain amount of compound solution was added to the solution, and after stirring for a period of time, the fluorescence intensity of the mixed solution was tested. The compound was continuously added and tested until the fluorescence in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com