Preparation method of three-dimensional carbon-boron nitride nanometer material
A nanomaterial, boron nitride technology, applied in chemical instruments and methods, inorganic chemistry, alkali metal compounds, etc., to achieve uniform morphology, simple method and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
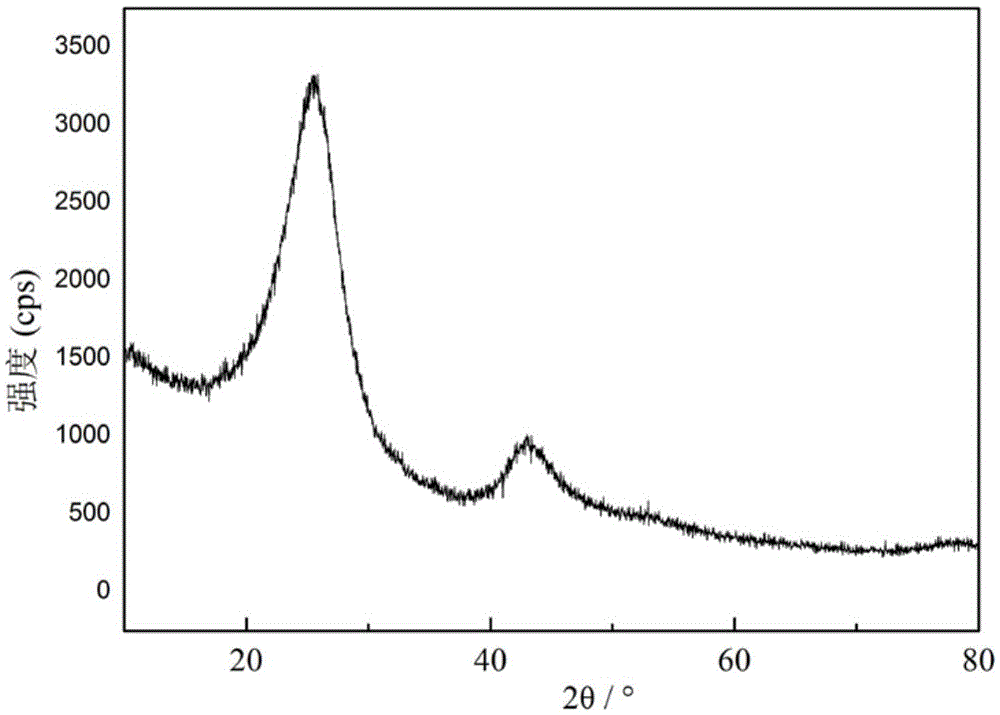
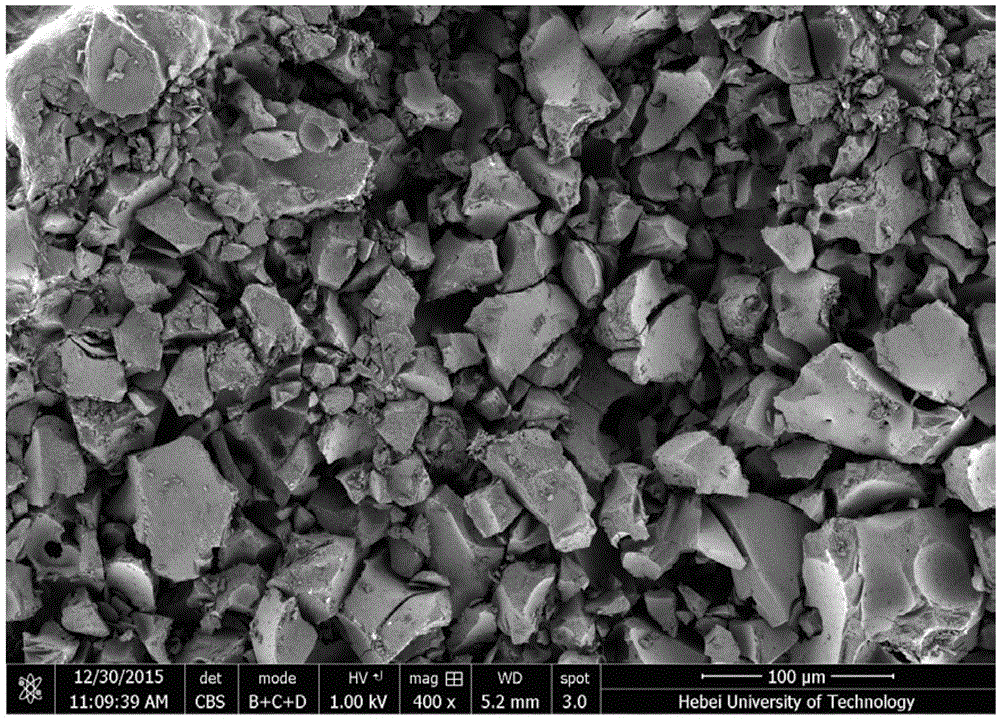
Image
Examples
Embodiment 1
[0033] (1) Weigh 20.9g of diboron trioxide and 29.8g of triethanolamine, and mix the two into a 50ml water-heated tank. At this time, the boron / nitrogen molar ratio of diboron trioxide and triethanolamine in the reaction material is 3:1, and the volume of the mixture accounts for about 80% of the capacity of the inner tank. Add a magnet and stir rapidly on a magnetic stirrer for 1 hour to fully mix the reactants;
[0034] (2) Place the reactor liner equipped with the reaction material in step 1 in the reactor, seal it, and heat it at 180° C. for 10 hours to carry out solvent heat treatment (triethanolamine plays the role of reactant and solvent at the same time), and then let The reaction kettle is naturally cooled to room temperature, and the mixed colloid of diboron trioxide-triethanolamine is obtained after unloading the kettle;
[0035] (3) Take 2.52 grams of boric acid and 2.47 grams of melamine and add them to 100 ml of deionized water respectively, stir for 1 hour and th...
Embodiment 2、 example 3
[0041] In embodiment 1 step (1), the weighing amount of diboron trioxide is respectively determined as: 6.96g, 2.32g, is about to change the consumption of diboron trioxide and triethanolamine into respectively according to boron atom and nitrogen atom molar ratio as 1:1 and 1:3 were mixed and added to the inner tank of the reactor, and other operations were the same as in Example 1, and the obtained product was the same as in Example 1.
Embodiment 4、 example 5
[0043] The weighing amount of boric acid in the embodiment 1 step (3) is set as 0.5g, 5g respectively; The amount of maintaining melamine is constant. Promptly only change the concentration of boric acid in the reactant to contain 0.005 gram, 0.05 gram of boric acid in every milliliter of water respectively, keep other each operation all identical with embodiment 1, the product that obtains is with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mesopore diameter | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com