Method for preparing C.I. pigment red 179
A compound and polar technology, applied in the direction of naphthalene dicarboxamide dyes/phthalimide dyes, etc., can solve the problems of large consumption of acid and alkali, lengthy reaction steps, and many kinds of chemical raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In a 500mL round bottom flask, add cesium hydroxide monohydrate (50.4g, 0.30mol), 1,8-diazabicycloundec-7-ene (76.0g, 0.50mol) and o-dichlorobenzene (180g ), under nitrogen protection, heated to 100°C under stirring, and then added N-methyl-1,8-naphthalimide (25.5g, 0.60mol) when the solid was completely dissolved. Thin-plate chromatography (TLC) tracked until the raw material point completely disappeared (about 12 hours), stopped heating, naturally cooled to room temperature, added methanol to dilute and then filtered, and the filter cake was washed with methanol until there was no o-dichlorobenzene smell. After drying, a dark red solid (21.5 g) was obtained, yield 80%.
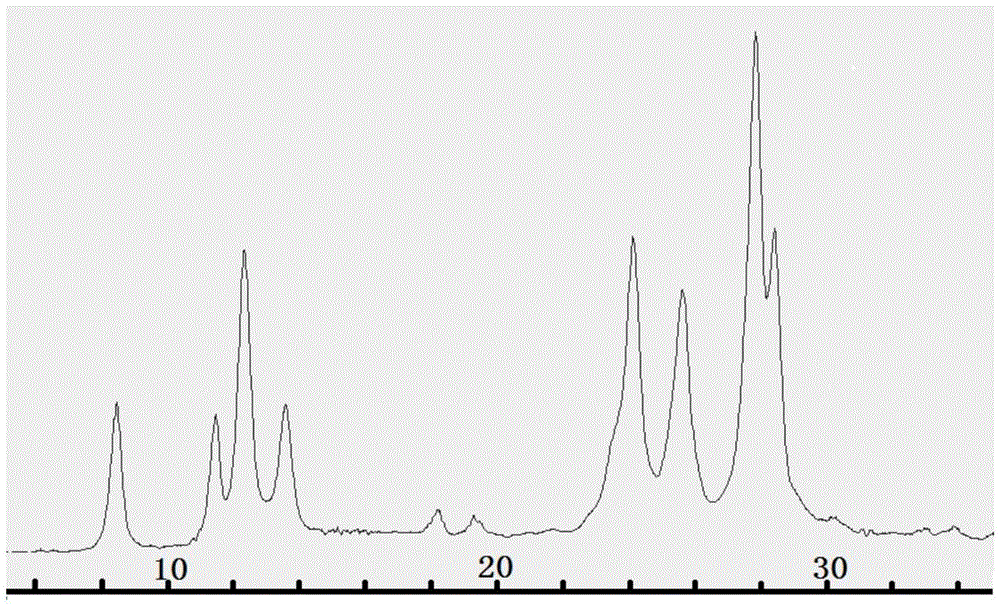
[0034]Adopt TOF-SIMS mass spectrometer (ION-TOFGmbH company) to measure the mass spectrum data of this dark red solid and the attribution of each ion peak as follows: M=418, molecular ion peak; M=404, [M-14] - (M-CH 2 ); M=390, [M-28] - (M-2xCH 2 ); M=375, [M-43] - (M-2xCH 2 –O+H); M=345, [M-73]...
Embodiment 2
[0036] Add cesium hydroxide monohydrate (67.2g, 0.40mol), 1,8-diazabicycloundec-7-ene (60.8g, 0.40mol) and 1,2,4- Trichlorobenzene (200g), under nitrogen protection, heated to 100°C under stirring, and then added N-methyl-1,8-naphthoimide (25.5g, 0.60mol) after the solid was completely dissolved. After adding, the temperature was raised to React at 190°C, follow TLC until the raw material point disappears (about 10 hours), stop heating, cool to room temperature naturally, add ethanol to dilute and filter, and wash the filter cake with ethanol until there is no 1,2,4-trichlorobenzene smell. After drying, a dark red solid (22.0 g) was obtained with a yield of 80%. The mass spectral data of the dark red solid measured by a TOF-SIMS mass spectrometer is the same as in Example 1.
Embodiment 3
[0037] Embodiment 3 (comparative example)
[0038] Refer to the literature TheJournaloforganicchemistry, 2001, 66(1):94-98. The method described in the preparation of N,N'-dimethyl-3,4,9,10-perylenetetracarboximide.
[0039] In a 100 mL round-bottomed flask, potassium tert-butoxide (8.08 g, 0.072 mol), 1,5-dioxabicyclo[4.3.0]non-5-ene (12.0 g, 0.096 mol) and diethylene glycol di Methyl ether (25mL), under the protection of nitrogen, heated to 130°C, when the solid was completely dissolved, add N-methyl-1,8-naphthoimide (5.08g, 0.12mol), after the addition, react for 3h, stop Heated, cooled to room temperature, diluted with DMF and filtered, and the filter cake was washed with DMF. After drying, a dark red solid (3.7 g) was obtained, yield 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com