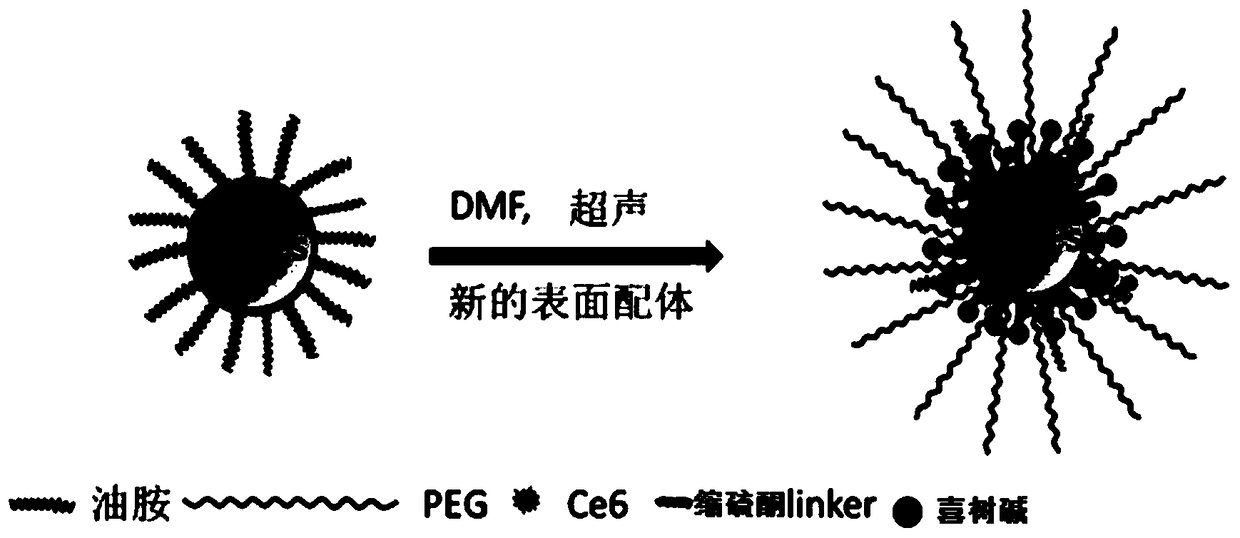
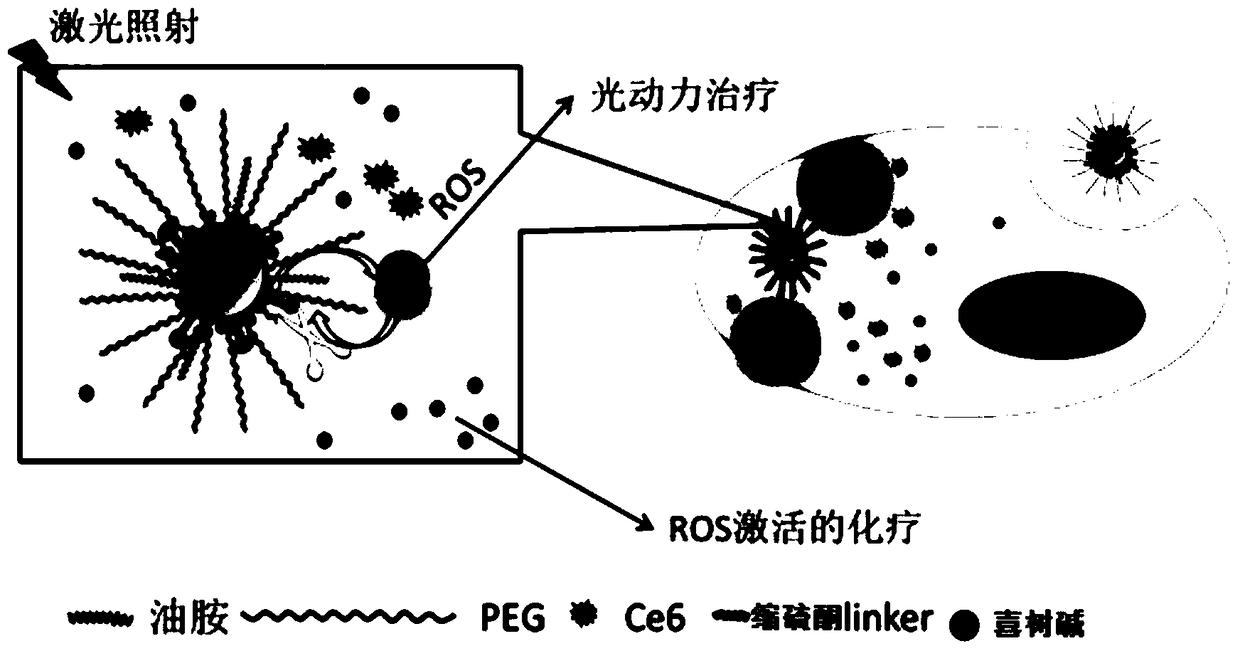
A ROS-responsive nano-drug delivery system and its preparation method and application
A delivery system and nano-drug technology, which is applied in the field of nano-drug delivery system and its preparation, can solve the problems of drug release that are rarely studied, and achieve the effects of good stability, good biocompatibility, and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. The synthesis steps of up-conversion material UCNPs are as follows:
[0055] 4mmol CF 3 COONa and 2mmol (total amount) Ln(CF 3 COO) 3 (Ln: 78mol%Y+20mol%Yb+1.6mol%Er+0.4mol%Tm) was added to a 100mL three-necked bottle, and 20mL oleylamine was added. Stir and heat to 120°C to remove moisture and oxygen, and stir for 1 h with nitrogen gas. During this period, the state of nitrogen gas has been kept, and when the reaction solution turns transparent, the temperature is raised to 320°C, and the temperature rises by 10°C per minute. After rising to 320°C, the reaction was stirred for 1 hour, and the reaction ended. When the temperature dropped to room temperature, excess oleylamine was washed away with hexane oxide and chloroform, and vacuum-dried.
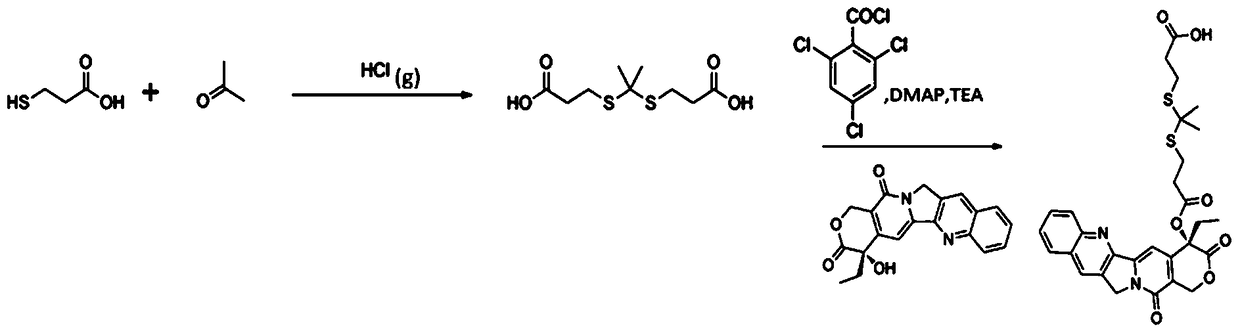
[0056] 2. The steps of synthesizing ROS-sensitive thioketal linker (TL) are as follows:
[0057] Anhydrous 3-mercaptopropionic acid (5.2g, 49.1mmol) and anhydrous acetone (5.8g, 98.2mmol) were saturated with dry HCl gas ...
Embodiment 2
[0064] 1. The synthesis steps of the up-conversion material UCNPs are the same as in Example 1, and will not be repeated here
[0065] 2. The steps of the ROS-sensitive thioketal linker (TL) are the same as those in Example 1, and will not be repeated here.
[0066] 3. The experimental steps of synthesizing TL-DOX are as follows:
[0067] TL (252.1mg, 1.0mmol) was dissolved in anhydrous 10mL DMF, followed by adding triethylamine (TEA, 303.6mg, 3.0mmol), 2,4,6-trichlorobenzoyl chloride (241.9mg, 1.0mmol) , dimethylaminopyridine (DMAP, 24.4 mg, 0.2 mmol), stirred for 10 min. Doxorubicin (DOX, 271.76mg, 0.5mmol) dissolved in 10mL of DMF was added, and the reaction was stirred at room temperature for 24h. The reaction was quenched with water, and the crude product was passed through a silica gel column to obtain pure product.
[0068] 4. The process of assembling Ce6-DOX-UCNPs is as follows:
[0069] Add TL-DOX (0.06mmol), mPEG-COOH (0.15mmol) and UCNPs (10mg) to 3mL DMF, add ...
Embodiment 3
[0071] 1. The synthesis steps of UCNPs, an up-conversion material, are the same as those in Example 1, and will not be repeated here.
[0072] 2. The steps of the ROS-sensitive thioketal linker (TL) are the same as those in Example 1, and will not be repeated here.
[0073] 3. The experimental steps for synthesizing TL-CPT are the same as those in Example 1, and will not be repeated here.
[0074] 4. The process of assembling PheA-CPT-UCNPs is as follows:
[0075] TL-CPT (0.06mmol), PheA (0.06mmol), mPEG-COOH (0.15mmol) and UCNPs (10mg) were added to 3mL DMF, ultrasonicated at 150W for 15min, stirred at room temperature for 1h and centrifuged three times (11250g, 10min / time) to collect the solid Substances were washed with HEPES buffer solution and resuspended.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com