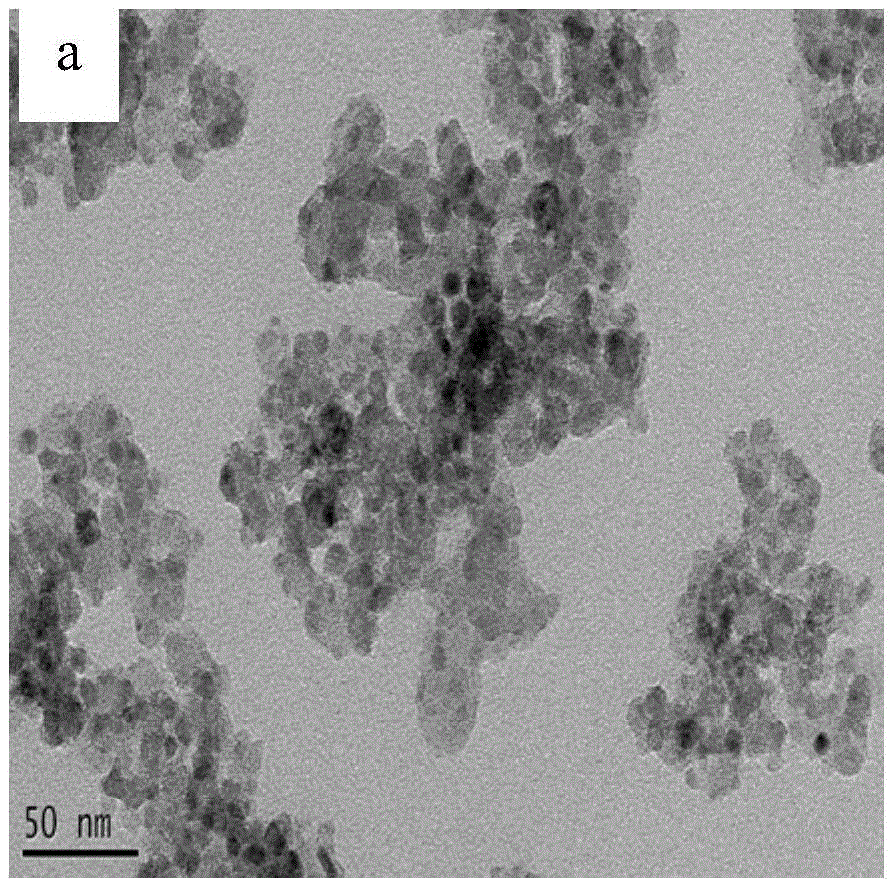
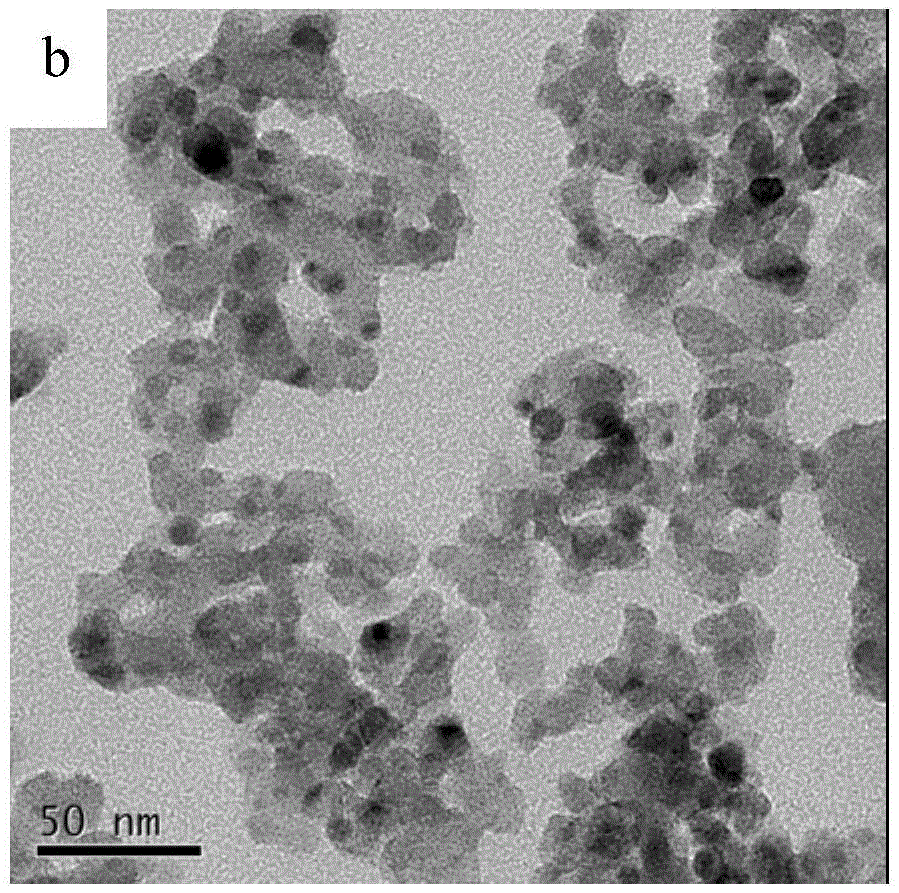
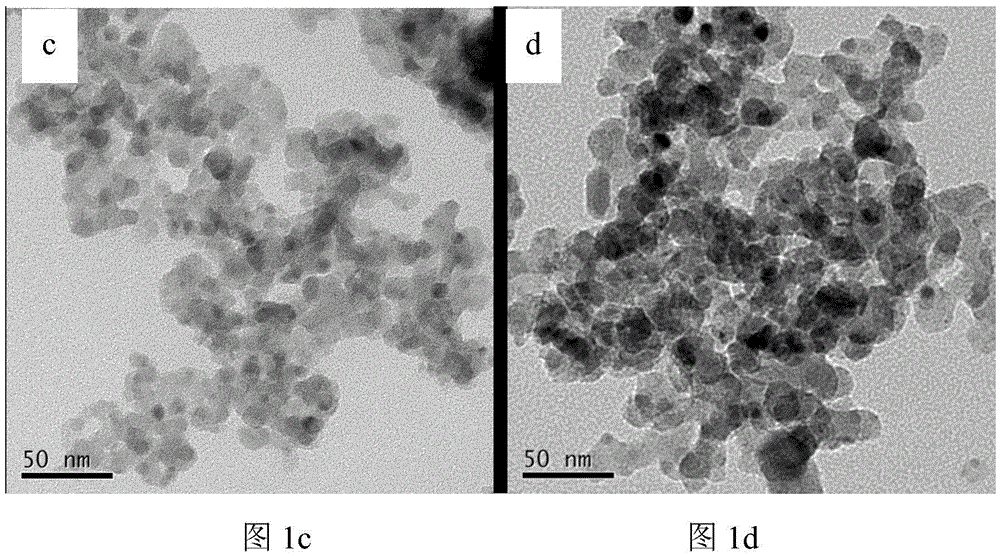
Visible-light responded compound catalyst for degrading organic pollutants in salt-containing wastewater and preparation method of visible-light responded compound catalyst
A technology of organic pollutants and composite catalysts, applied in water pollutants, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of catalytic activity reduction, surface active site occupation, disappearance, etc. problem, to achieve the effect of simple method, mild condition and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Catalyst preparation
[0041] Get 0.5g of silicon dioxide with hydrophilic surface (specific surface area 180m 2 / g), 200mL of absolute ethanol and 2mL of NaOH aqueous solution (10g / L) were placed in a flask, stirred and adsorbed in a 30°C water bath. After adsorption equilibrium (more than 12 hours), a stable adsorption water layer is formed on the surface of graphene oxide.
[0042] Another 50 mL of absolute ethanol in which 2.15 g of tetrabutyl titanate and 9 mg of ferric nitrate were dissolved was dropped into the adsorption equilibrium system drop by drop using a constant pressure funnel. Butyl titanate undergoes hydrolysis reaction in the adsorption water layer, and iron ions undergo precipitation reaction with hydroxide ions in the adsorption water layer, finally generating Fe(OH) 3 -TiO 2 , after the completion of the reaction for 5 hours, a suspension system containing composite particles was obtained.
[0043] Add the suspended system after the reactio...
Embodiment 2
[0053] (1) Catalyst preparation
[0054] Get 0.5g of silicon dioxide with hydrophilic surface (the specific surface area is 180m 2 / g), 200mL of absolute ethanol and 2mL of NaOH aqueous solution (10g / L) were placed in a flask, stirred and adsorbed in a 30°C water bath. After adsorption equilibrium (more than 12 hours), a stable adsorption water layer is formed on the surface of graphene oxide.
[0055] Another 50 mL of absolute ethanol in which 2.15 g of tetrabutyl titanate and 27 mg of ferric nitrate were dissolved was dropped into the adsorption equilibrium system drop by drop using a constant pressure funnel. Butyl titanate undergoes hydrolysis reaction in the adsorption water layer, and iron ions undergo precipitation reaction with hydroxide ions in the adsorption water layer, finally generating Fe(OH) 3 -TiO 2 , after the completion of the reaction for 5 hours, a suspension system containing composite particles was obtained.
[0056] Add the suspension system after th...
Embodiment 3
[0066] (1) Catalyst preparation
[0067] Get 0.5g of silicon dioxide with hydrophilic surface (the specific surface area is 180m 2 / g), 200mL of absolute ethanol and 2mL of NaOH aqueous solution (10g / L) were placed in a flask, stirred and adsorbed in a 30°C water bath. After adsorption equilibrium (more than 12 hours), a stable adsorption water layer is formed on the surface of graphene oxide.
[0068] Another 50 mL of absolute ethanol in which 2.15 g of tetrabutyl titanate and 54 mg of ferric nitrate were dissolved was dropped into the adsorption equilibrium system drop by drop using a constant pressure funnel. Butyl titanate undergoes hydrolysis reaction in the adsorption water layer, and iron ions undergo precipitation reaction with hydroxide ions in the adsorption water layer, finally generating Fe(OH) 3 -TiO 2 , after the completion of the reaction for 5 hours, a suspension system containing composite particles was obtained.
[0069] Add the suspended system after the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com