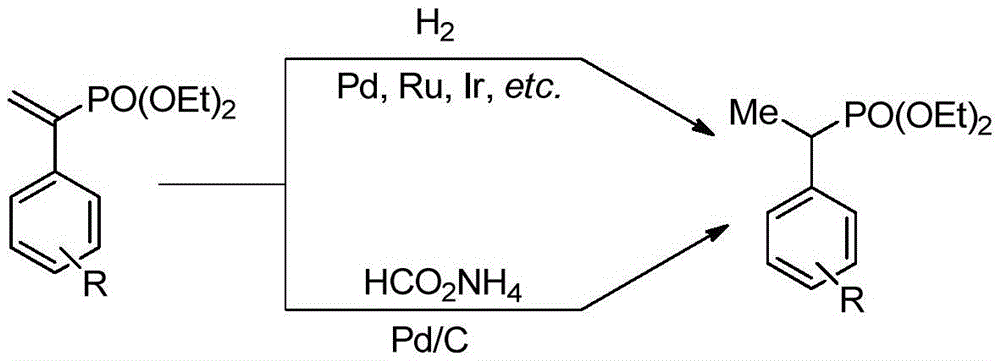
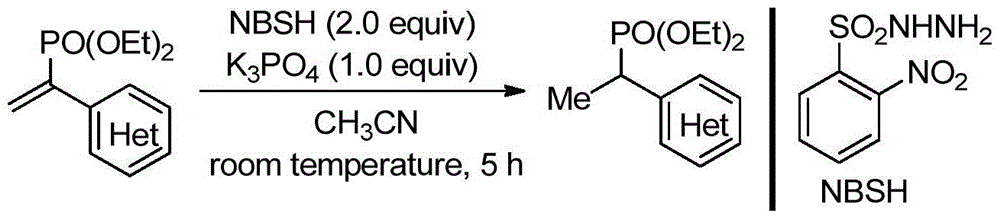
Method for synthesizing alpha-aryl ethyl phosphonate
A technology of aryl ethyl phosphonate and aryl vinyl phosphonate, which is applied in the field of synthesizing α-aryl ethyl phosphonate, can solve the problems of heavy metal residues in hydrogenation products, and achieve strong functional group compatibility and reaction Mild conditions and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 0.3mmol of diethyl α-(4-chlorophenyl)vinylphosphonate, 0.6mmol of o-nitrobenzenesulfonyl hydrazide, and 0.3mmol of potassium carbonate to a Schlenk reaction tube (10mL), vacuumize and release N 2 Cycle three times. in N 2 Under protection, add 2.0 mL of anhydrous acetonitrile, stir at room temperature for 5 h; after TLC confirms that the reaction is complete, add 5 mL of water to the reaction system, extract 3 times with 30 mL of ethyl acetate (10 mL*3), combine the organic phases, and wash with anhydrous sulfuric acid Sodium drying; then filter to remove sodium sulfate, and distill through a rotary evaporator to obtain an organic primary product. Then, the above-mentioned organic primary product is separated by column chromatography with a mixture of petroleum ether and ethyl acetate with a volume ratio of 1:1 as an eluent, and finally α-(4-chlorophenyl) ethyl acetate is obtained by distillation with a rotary evaporator. Diethyl phosphonate 80.3 mg, yield 97%. T...
Embodiment 2
[0023] Add 0.3mmol of diethyl α-(3-chlorophenyl)vinylphosphonate, 0.6mmol of o-nitrobenzenesulfonyl hydrazide, and 0.3mmol of potassium phosphate into a Schlenk reaction tube (10mL). 2 Cycle three times. in N 2 Under protection, add 2.0 mL of anhydrous acetonitrile, stir at room temperature for 5 h; after TLC confirms that the reaction is complete, add 5 mL of water to the reaction system, extract 3 times with 30 mL of ethyl acetate (10 mL*3), combine the organic phases, and wash with anhydrous sulfuric acid Sodium drying; then filter to remove sodium sulfate, and distill through a rotary evaporator to obtain an organic primary product. Then, the above-mentioned organic primary product is separated by column chromatography with a mixture of petroleum ether and ethyl acetate with a volume ratio of 1:1 as an eluent, and finally α-(3-chlorophenyl) ethyl acetate is obtained by distillation with a rotary evaporator. Diethyl phosphonate 74.5 mg, yield 90%. The analysis data is as...
Embodiment 3
[0026] Add 0.3mmol of diethyl α-(4-methylphenyl)vinylphosphonate, 0.6mmol of o-nitrobenzenesulfonyl hydrazide, and 0.3mmol of potassium phosphate into a Schlenk reaction tube (10mL), vacuumize and release N 2 Cycle three times. in N 2 Under protection, add 2.0 mL of anhydrous acetonitrile, stir at room temperature for 5 h; after TLC confirms that the reaction is complete, add 5 mL of water to the reaction system, extract 3 times with 30 mL of ethyl acetate (10 mL*3), combine the organic phases, and wash with anhydrous sulfuric acid Sodium drying; then filter to remove sodium sulfate, and distill through a rotary evaporator to obtain an organic primary product. Then the above-mentioned organic primary product is separated by column chromatography with a mixture of petroleum ether and ethyl acetate with a volume ratio of 1:1 as an eluent, and finally α-(4-methylphenyl) is obtained by distillation with a rotary evaporator. Diethyl ethylphosphonate 76.0 mg, yield 99%. The analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com