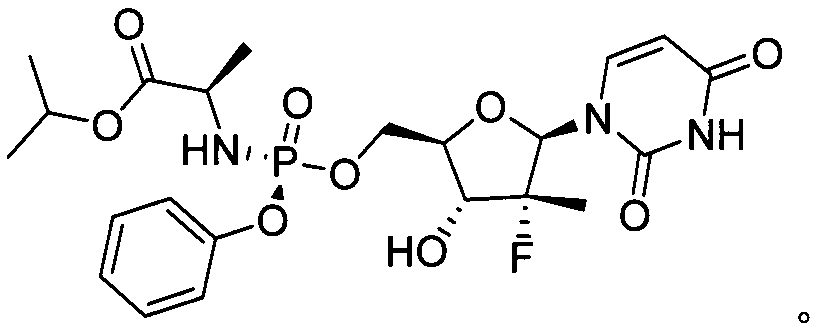
High-yield synthesis method of sofosbuvir and sofosbuvir prepared with method
A technology of sofosbuvir and a synthetic method, which is applied in the field of sofosbuvir, a synthetic method and the sofosbuvir prepared by the sofosbuvir, can solve the problems of rising cost and reduced yield, and achieves improved yield and reduced yield. The number of reaction steps and the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
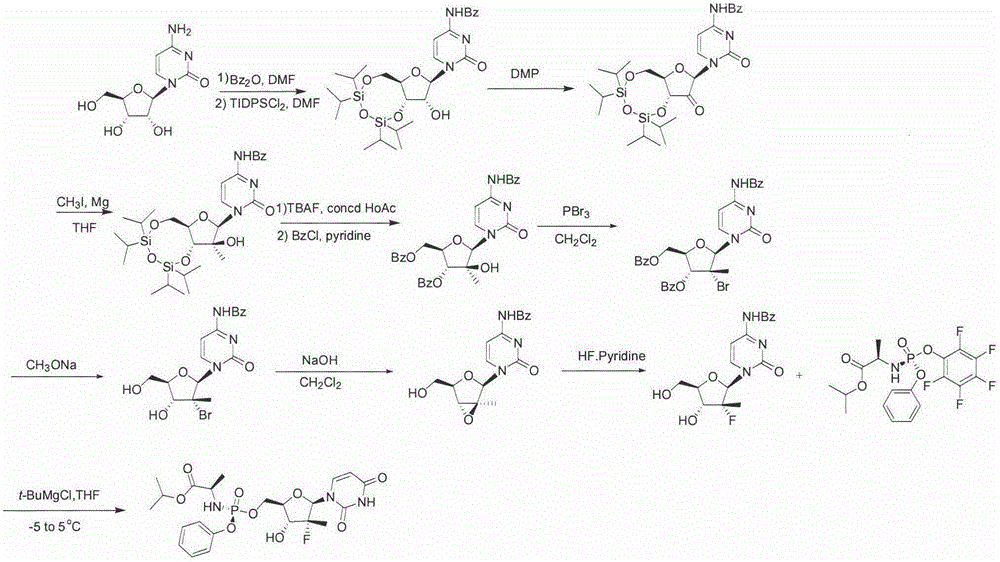
[0029] The present embodiment provides a kind of synthetic method of high-yield sofosbuvir, such as figure 1 As shown, it includes the following steps:
[0030] (a) benzoic anhydride (Bz 2 O) Dissolve in 5L of N,N-dimethylformamide (DMF), stir and react at room temperature for 4 hours, then add water to quench the reaction, extract 3 times with 2L of ethyl acetate, add anhydrous sodium sulfate to dry , filtered and then rotary evaporated to remove ethyl acetate to obtain the first product; redissolve it in 5LDMF, add 1.1eq. of TIDPSCl 2 , stirred at room temperature for 8 hours, then added water to quench the reaction, extracted 3 times with 2L of ethyl acetate, combined the organic phases, added anhydrous sodium sulfate to dry, filtered, and rotary evaporated to remove ethyl acetate to obtain the second product;
[0031] (b) Dissolve the second product and 1.5eq. of dimethyl phthalate in 5L of dichloromethane, stir and react at room temperature for 5 hours, remove the dichl...
Embodiment 2
[0040] This implementation provides a kind of synthetic method of high-yield sofosbuvir, its synthetic steps are basically the same as in Example 1, the difference is: in step (h), also add 0.05eq. by benzoyl peroxide and The molar ratio of dicumyl peroxide is 1:1 as a catalyst to finally obtain 0.3eq. of sofosbuvir.
Embodiment 3
[0042] The present embodiment provides a kind of synthetic method of high-yield sofosbuvir, such as figure 1 As shown, it includes the following steps:
[0043] (a) 1kg of cytidine (molecular weight is 243.22, 4.11mol) and 1eq. of benzoic anhydride (Bz 2 O) Dissolve in 5L of N,N-dimethylformamide (DMF), stir and react at room temperature for 3 hours, then add water to quench the reaction, extract 5 times with 2L of ethyl acetate, add anhydrous sodium sulfate to dry , filtered and then rotary evaporated to remove ethyl acetate to obtain the first product; redissolve it in 5LDMF, add 1.05eq. of TIDPSCl 2 , stirred at room temperature for 7 hours, then added water to quench the reaction, extracted 5 times with 2L of ethyl acetate, added anhydrous sodium sulfate to dry, filtered, and rotary evaporated to remove ethyl acetate to obtain the second product;
[0044] (b) Dissolve the second product and 1.8eq. of dimethyl phthalate in 5L of dichloromethane, stir and react at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com