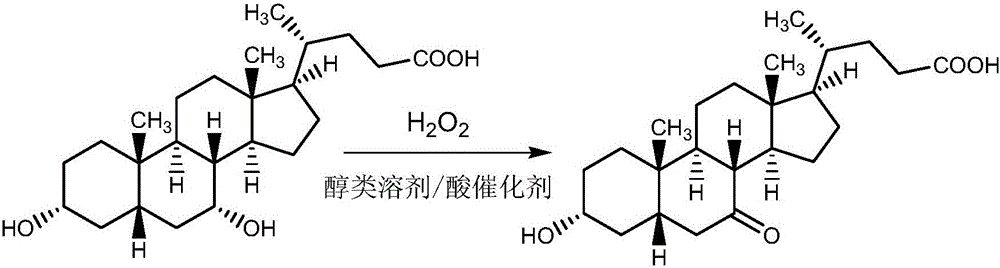
Preparation method of 3Alpha-hydrol-7-oxo-5Beta-cholanic acid
A technology of cholanoic acid and chenodeoxycholic acid, which is applied in the directions of steroids and organic chemistry, can solve the problems of difficult combination and optimization, poor compatibility with organic solvents, poor selectivity, etc., and achieves broad development and application prospects. The effect of mild reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation and purification of 3α-hydroxy-7-oxo-5β-cholanic acid.
[0032] (1) Preparation of 3α-hydroxy-7-oxo-5β-cholanic acid:
[0033] Add chenodeoxycholic acid (50g), methanol (300g) and citric acid (5g) into a three-necked flask, heat to 50°C under mechanical stirring, and keep it for 30min; after cooling to 5°C, add hydrogen peroxide solution dropwise ( 16g, 28wt%), and the dropping temperature was controlled within 15°C. After the dropwise addition was completed, the temperature was kept at 10°C for 5 hours; after the heat preservation reaction was completed, methanol (200g) was recovered and water (500g) was added, stirred for 2 hours, and filtered , washed with water, and dried to obtain a crude product of 3α-hydroxy-7-oxo-5β-cholanic acid.
[0034] (2) Purification of 3α-hydroxy-7-oxo-5β-cholanic acid:
[0035] Add the crude product prepared in step (1) to a mixed solvent of isopropanol and ethyl acetate (isopropanol: ethyl acetate = 1:3 (v / v)), ...
Embodiment 2
[0036] Example 2: Preparation and purification of 3α-hydroxy-7-oxo-5β-cholanic acid.
[0037] (1) Preparation of 3α-hydroxy-7-oxo-5β-cholanic acid:
[0038] Add chenodeoxycholic acid (80g), ethanol (640g) and tartaric acid (8g) into a three-necked flask, heat to 45°C under mechanical stirring, and keep it for 30min; after cooling to 5°C, add dropwise hydrogen peroxide aqueous solution (26g , 28wt%), and the dropping temperature was controlled within 15°C. After the dropwise addition was completed, the reaction was incubated at 11°C for 4h; Washed with water and dried to obtain the crude product of 3α-hydroxy-7-oxo-5β-cholanic acid.
[0039] (2) Purification of 3α-hydroxy-7-oxo-5β-cholanic acid:
[0040] Add the crude product prepared in step (1) to a mixed solvent of isopropanol and ethyl acetate (isopropanol: ethyl acetate = 1:3 (v / v)), heat and reflux for 30 minutes under mechanical stirring; the reflux is completed Finally, add activated carbon for decolorization and fil...
Embodiment 3
[0041] Example 3: Preparation and purification of 3α-hydroxy-7-oxo-5β-cholanic acid.
[0042] (1) Preparation of 3α-hydroxy-7-oxo-5β-cholanic acid:
[0043] Add chenodeoxycholic acid (130g), isopropanol (900g) and sulfuric acid (13g) into a three-necked flask, heat to 30°C under mechanical stirring, and keep it for 1h; after cooling to 2°C, add hydrogen peroxide solution dropwise (42g, 28wt%), and the dropping temperature was controlled within 15°C. After the dropwise addition was completed, the reaction was incubated at 12°C for 3h; Filter, wash with water, and dry to obtain the crude product of 3α-hydroxy-7-oxo-5β-cholanic acid.
[0044] (2) Purification of 3α-hydroxy-7-oxo-5β-cholanic acid:
[0045] Add the crude product prepared in step (1) to a mixed solvent of isopropanol and ethyl acetate (isopropanol: ethyl acetate = 1:3 (v / v)), heat and reflux for 30 minutes under mechanical stirring; the reflux is completed Finally, add activated carbon for decolorization and filter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com