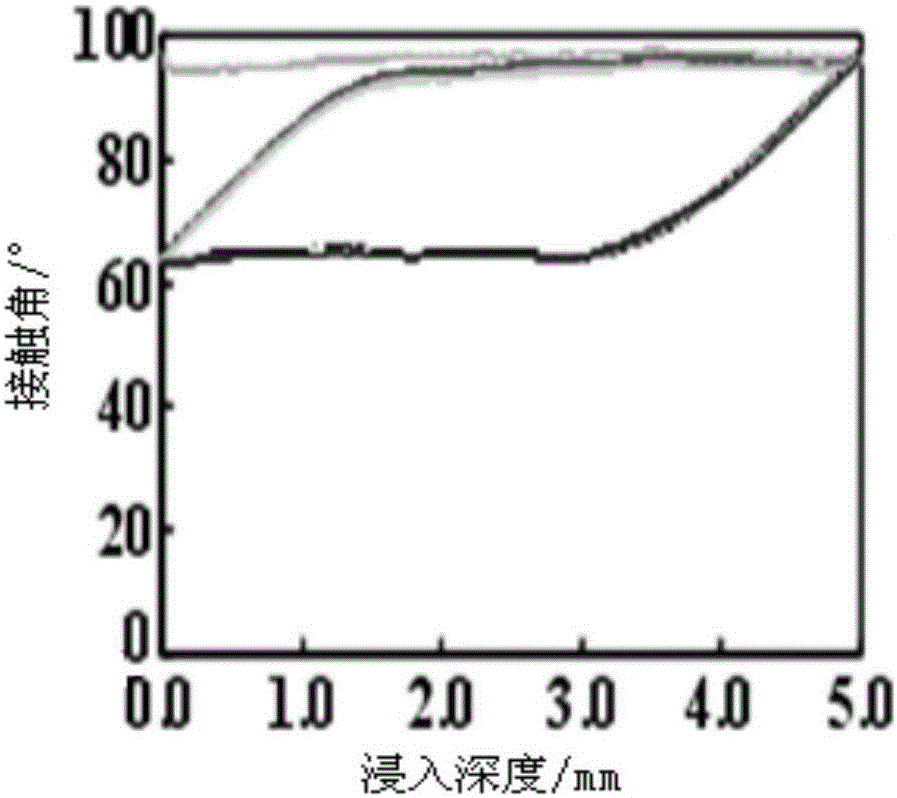
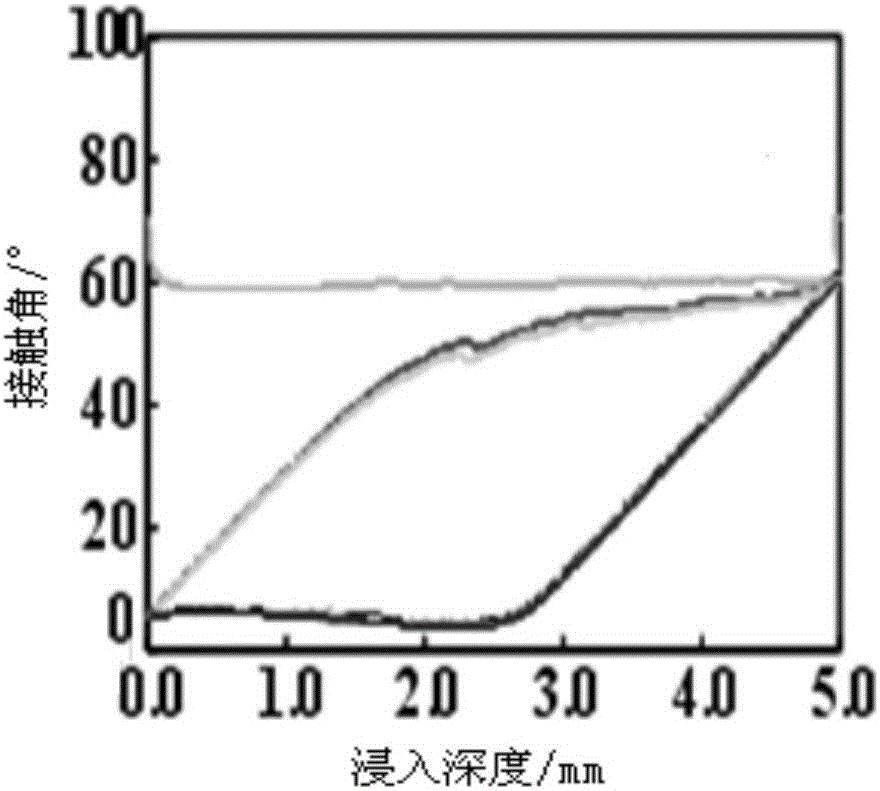
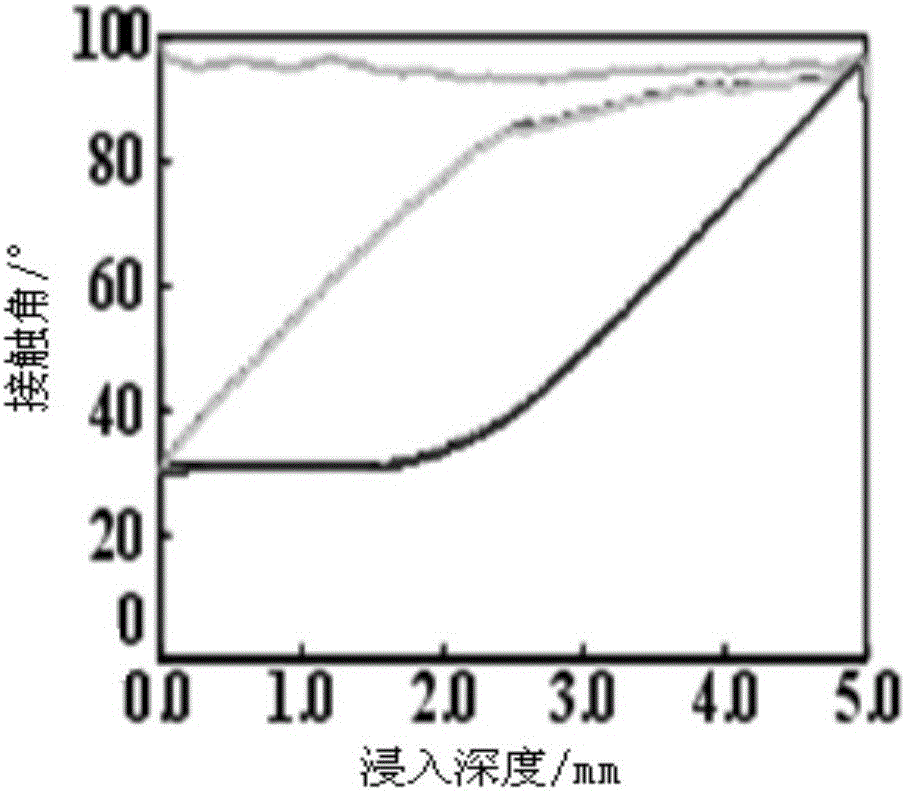
Preparation method of phosphorylcholine bionic coating
A phosphorylcholine and coating technology, which is applied in the field of material surface science and biomedical polymer materials, can solve the problem of difficulty in obtaining a high-content phosphorylcholine group surface, and the rigidity of the fixed group hindering the phosphorylcholine group. Orientation migration, limiting biocompatibility and other issues, to achieve the effect of improved hydrophilicity and blood compatibility, simple method and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] This embodiment includes the following steps:
[0039] Step 1. Under nitrogen protection conditions, dissolve 16mmol of 2-methacryloyloxyethyl phosphorylcholine monomer and 4mmol of 2-aminoethyl methacrylate hydrochloride monomer in distilled water in 0.1mmol of initiator Carry out free radical polymerization under the influence of free radical polymerization. The reaction temperature of free radical polymerization is 70°C, and the reaction time is 12h. Freeze-drying under low temperature, obtain the phosphorylcholine polymer containing amino group; Described initiator is potassium persulfate;
[0040] Step 2. Dissolve 1 mg of the amino group-containing phosphorylcholine polymer described in step 1 in 1 mL of methanol to obtain a polymer solution, then add 15 μL of glutaraldehyde aqueous solution to the polymer solution, mix well, and obtain a mixed Solution; The mass concentration of described glutaraldehyde aqueous solution is 50%;
[0041] Step 3. Apply the mixed s...
Embodiment 2
[0044] Example 2 is the same as Example 1, except that the polycarbonate film obtained in step 3 with a phosphorylcholine biomimetic coating on its surface is heated for 12 hours under a vacuum condition of 110° C., and washed with distilled water Finally, the phosphorylcholine biomimetic coating after hydrophobic treatment was obtained.
Embodiment 3
[0056] This embodiment includes the following steps:
[0057] Step 1. Under the condition of nitrogen protection, 14 mmol of acryloyloxyethyl phosphorylcholine monomer and 6 mmol of 2-aminoethyl methacrylate hydrochloride monomer are dissolved in distilled water and then carried out under the action of 0.2 mmol of initiator Free radical polymerization, the reaction temperature of free radical polymerization is 60°C, and the reaction time is 20h. After the reaction, the reaction solution is concentrated, then dialyzed with a dialysis bag with a molecular weight cut-off of 6000D to 8000D, and then freeze-dried at -50°C , to obtain phosphorylcholine polymers containing amino groups; the initiator is ammonium persulfate;
[0058] Step 2. Dissolve 0.5 mg of the amino group-containing phosphorylcholine polymer in 1 mL of methanol to obtain a polymer solution, then add 10 μL of glutaraldehyde aqueous solution to the polymer solution, and mix well to obtain Mixed solution; the mass c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com