Preparation method of catalyst for photo-catalytically splitting water to produce hydrogen
A technology of photolysis of water to produce hydrogen and catalysts, applied in the field of nanomaterials, can solve the problems of weak visible light response, low hydrogen production efficiency, and high cost, and achieve the effects of low price, high hydrogen production efficiency, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
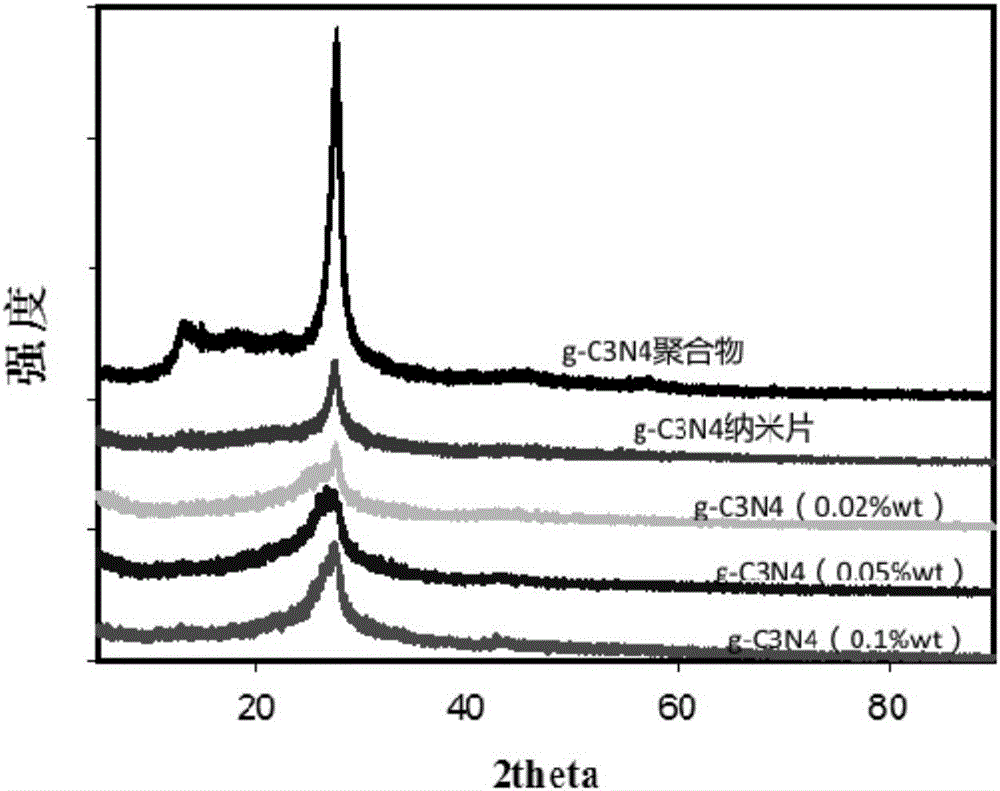
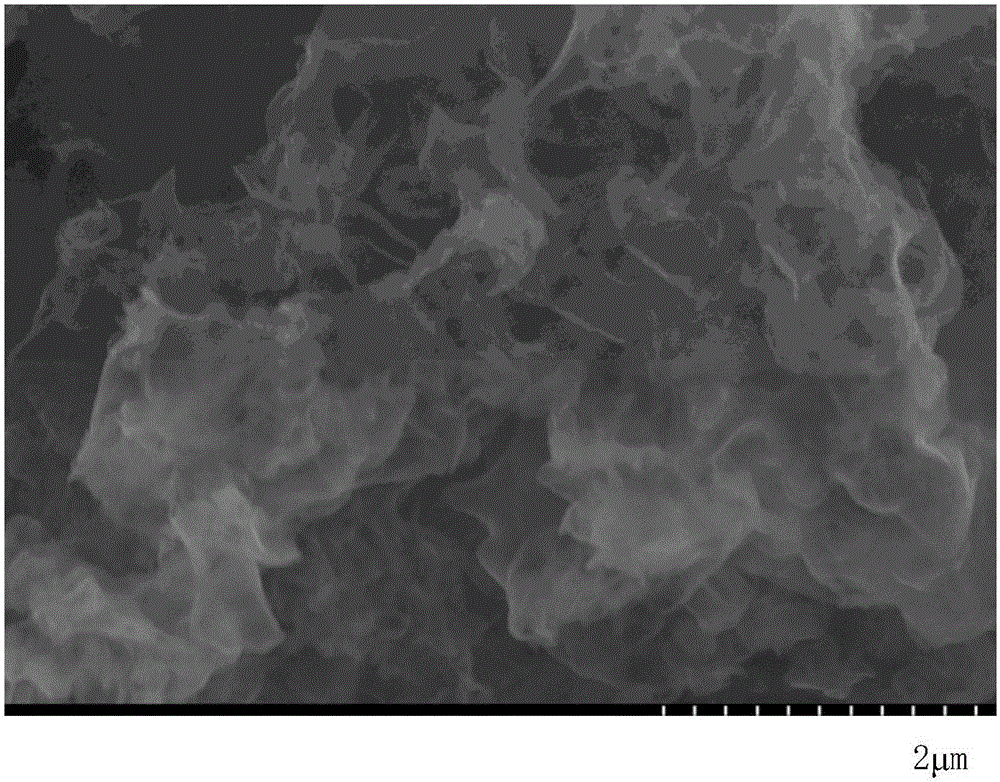
[0022] Add 10 g of urea into a 50 mL ceramic crucible with a lid, place the crucible in a muffle furnace, raise it from room temperature to 550 °C at a rate of 2.5 °C / min, and keep the temperature constant for 2 hours. After calcination, cool to room temperature, grind the sample in an agate mortar to obtain a yellow powder, namely g-C 3 N 4 polymer material. Under the condition of nitrogen protection, weigh 108mgg-C 3 N 4 Add the polymer material to 120mL of freshly dried tetrahydrofuran solution, ultrasonicate for 5min, then add 0.55g of metal lithium and 5.08g of naphthalene to the dispersion, the solution turns dark green and then keep stirring for 1h, then use an automatic injector to 1-Bromododecane was added to the above solution at a speed of 100°C until the green color of the solution disappeared, and the reaction was continued for 12 h under the protection of nitrogen. After the reaction, add ethanol to the above reactant to remove unreacted lithium metal, centri...
Embodiment 2
[0025] Add 10 g of urea into a 50 mL ceramic crucible with a lid, place the crucible in a muffle furnace, raise it from room temperature to 550 °C at a rate of 2.5 °C / min, and keep the temperature constant for 2 hours. After calcination, cool to room temperature, grind the sample in an agate mortar to obtain a yellow powder, namely g-C 3 N 4 polymer material. Under the condition of nitrogen protection, weigh 108mgg-C 3 N 4 Add the polymer material to 120mL of freshly dried tetrahydrofuran solution, ultrasonicate for 5min, then add 0.55g of metal lithium and 5.08g of naphthalene to the dispersion, the solution turns dark green and then keep stirring for 1h, then use an automatic injector to 1-Bromododecane was added to the above solution at a speed of 100°C until the green color of the solution disappeared, and the reaction was continued for 12 h under the protection of nitrogen. After the reaction, add ethanol to the above reactant to remove unreacted lithium metal, centri...
Embodiment 3
[0027] Add 10 g of urea into a 50 mL ceramic crucible with a lid, place the crucible in a muffle furnace, raise it from room temperature to 550 °C at a rate of 2.5 °C / min, and keep the temperature constant for 2 hours. After calcination, cool to room temperature, grind the sample in an agate mortar to obtain a yellow powder, namely g-C 3 N 4 polymer material. Under the condition of nitrogen protection, weigh 368mgg-C 3 N 4 Add the polymer material to 120mL of freshly dried tetrahydrofuran solution, ultrasonicate for 5min, then add 0.21g of metal lithium and 2.56g of naphthalene to the dispersion, the solution turns dark green, and then keep stirring for 1h, and then use an automatic injector at 10mL / h 1-Bromo-n-hexane was added to the above solution at a speed of 100°C until the green color of the solution disappeared, and the reaction was continued for 12 h under the protection of nitrogen. After the reaction, add ethanol to the above reactant to remove unreacted lithium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com