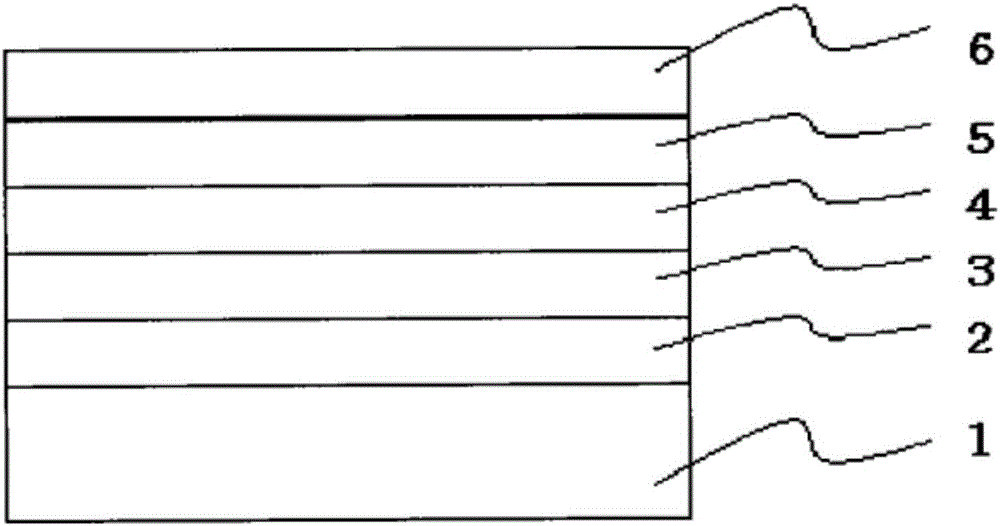
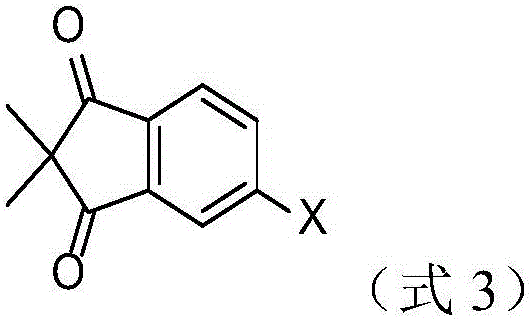
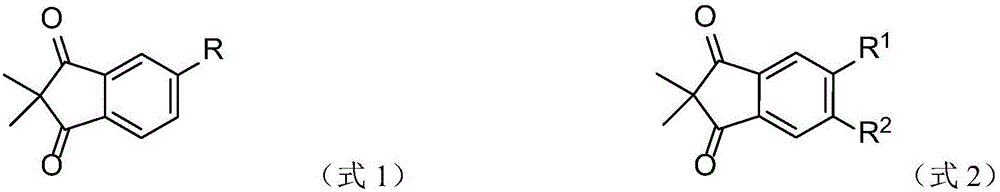2, 2-dimethyl-1, 3-indandione derivatives and organic electroluminescence device based on same
A technology of indanedione and dimethyl, applied in the field of organic electroluminescence devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Reaction is as shown in (formula 8):
[0078]
[0079] The specific steps of the reaction are as follows:
[0080] Under argon protection, 252 mg of 5-bromo-2,2-dimethyl-1,3-indanedione, 680 mg of 9,9-dimethylacridine, 15 mg of palladium acetate, 1.1 g of potassium tert-butoxide, 0.3 mL of a toluene solution of tri-tert-butylphosphine with a volume fraction of 10% and 10 mL of toluene to obtain the first mixed solution; the first mixed solution was heated to 90° C. and stirred under reflux After 48 hours, the second mixed solution was obtained; the second mixed solution was cooled to room temperature, and the organic solvent was distilled off to obtain the third mixed solution; a large amount of water and dichloromethane were added to the third mixed solution for extraction, and the extracted The final organic phase was dried with anhydrous sodium sulfate, filtered, and then the organic liquid phase was distilled off to obtain a crude product; the crude product was ...
Embodiment 2
[0084] Reaction is as shown in (formula 9):
[0085]
[0086] The specific steps of the reaction are as follows:
[0087] S01: Slowly add 4.7g (0.21mol) of sodium into 50mL (0.48mol) of absolute ethanol to make sodium ethylate; add the sodium ethylate to 60mL of methyl propionate and 52.8g (0.15mol) of 5, In the mixed solution of 6-dibromophthalic acid dimethyl ester, the temperature was raised to 80°C and stirred for 4 hours to obtain a light yellow viscous paste; then the methanol in the paste was distilled to , ethanol, and methyl propionate were removed, then suction filtered, the filter residue was washed with petroleum ether, and 40.5 g of a light yellow solid was obtained after vacuum drying.
[0088] S02: Add the light yellow solid to 100 mL of 3 mol / L hydrochloric acid solution, then raise the temperature to 90°C and stir until CO is generated 2 Cool until it is removed, then suction filter after cooling to obtain light yellow crystals, wash the light yellow crys...
Embodiment 3
[0095] Reaction is as shown in (formula 10):
[0096]
[0097] The specific steps of the reaction are as follows:
[0098] S1: Under argon protection, add 350 mg of 5,6-dibromo-2,2-dimethyl-1,3-indandione, 680 mg of phenoxazine, and acetic acid into a 100 mL reactor equipped with a reflux tube 15 mg of palladium, 1.1 g of potassium tert-butoxide, 0.3 mL of a toluene solution of tri-tert-butylphosphine with a volume fraction of 10%, and 10 mL of toluene to obtain the first mixed solution; the first mixed solution was heated to 90° C. and stirred under reflux After 48 hours, the second mixed solution was obtained; the second mixed solution was cooled to room temperature, and the organic solvent was distilled off to obtain the third mixed solution; a large amount of water and dichloromethane were added to the third mixed solution for extraction, and the extracted The final organic phase is dried and filtered with anhydrous sodium sulfate, and then the organic liquid phase is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com